How To Make A Cation Compound . How to make the chemical formulas for a merging cation and anion? Construct a proper formula for an ionic compound. Predict the charge of monatomic main group elements based on their. So far, we have discussed elements and. For example, when each sodium atom in a sample of sodium metal (group 1) gives up one electron to form a sodium cation, na +, and each chlorine atom in a sample of chlorine gas (group. Ionic compounds do not exist as molecules. How do you write a net ionic equation for the reaction of aqueous. A proper ionic formula has a cation and an anion in it; Recognize polyatomic ions in chemical formulas. Recognize that metals lose electrons to form cations and that nonmetals gain electrons to form anions. An ionic compound is never formed between two cations only or two anions only. Generate a proper name for an ionic compound. If the compound contains a cation of a transition metal with two possible charges, then a roman numeral or the corresponding suffix is used to indicate its charge. In the solid state, ionic compounds are in crystal.
from chem.libretexts.org
Recognize that metals lose electrons to form cations and that nonmetals gain electrons to form anions. Recognize polyatomic ions in chemical formulas. Generate a proper name for an ionic compound. For example, when each sodium atom in a sample of sodium metal (group 1) gives up one electron to form a sodium cation, na +, and each chlorine atom in a sample of chlorine gas (group. In the solid state, ionic compounds are in crystal. Ionic compounds do not exist as molecules. So far, we have discussed elements and. Construct a proper formula for an ionic compound. How do you write a net ionic equation for the reaction of aqueous. An ionic compound is never formed between two cations only or two anions only.
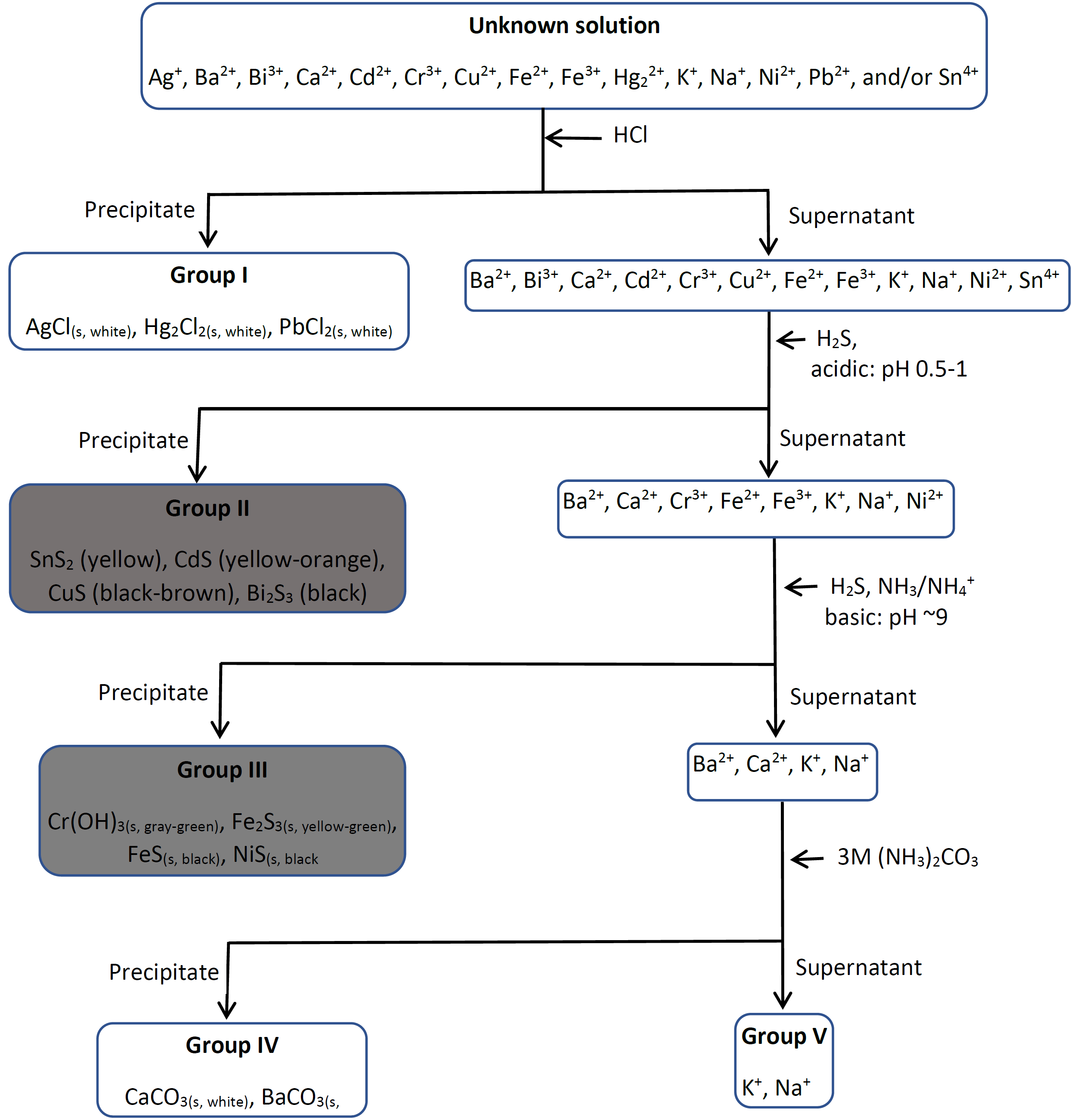
1.5 Separation of cations in groups Chemistry LibreTexts
How To Make A Cation Compound Recognize polyatomic ions in chemical formulas. In the solid state, ionic compounds are in crystal. Ionic compounds do not exist as molecules. Recognize that metals lose electrons to form cations and that nonmetals gain electrons to form anions. An ionic compound is never formed between two cations only or two anions only. If the compound contains a cation of a transition metal with two possible charges, then a roman numeral or the corresponding suffix is used to indicate its charge. Predict the charge of monatomic main group elements based on their. For example, when each sodium atom in a sample of sodium metal (group 1) gives up one electron to form a sodium cation, na +, and each chlorine atom in a sample of chlorine gas (group. Construct a proper formula for an ionic compound. How do you write a net ionic equation for the reaction of aqueous. A proper ionic formula has a cation and an anion in it; How to make the chemical formulas for a merging cation and anion? Recognize polyatomic ions in chemical formulas. Generate a proper name for an ionic compound. So far, we have discussed elements and.
From www.tes.com
Solubility of Anions and Cations Information Sheet Teaching Resources How To Make A Cation Compound How to make the chemical formulas for a merging cation and anion? So far, we have discussed elements and. Recognize polyatomic ions in chemical formulas. Recognize that metals lose electrons to form cations and that nonmetals gain electrons to form anions. For example, when each sodium atom in a sample of sodium metal (group 1) gives up one electron to. How To Make A Cation Compound.
From www.numerade.com
SOLVED Complete the table below by writing the symbols for the cation How To Make A Cation Compound Construct a proper formula for an ionic compound. For example, when each sodium atom in a sample of sodium metal (group 1) gives up one electron to form a sodium cation, na +, and each chlorine atom in a sample of chlorine gas (group. Recognize polyatomic ions in chemical formulas. So far, we have discussed elements and. Recognize that metals. How To Make A Cation Compound.
From brainly.in
Make a chart and write all chemical formula and name of compound using How To Make A Cation Compound An ionic compound is never formed between two cations only or two anions only. Ionic compounds do not exist as molecules. For example, when each sodium atom in a sample of sodium metal (group 1) gives up one electron to form a sodium cation, na +, and each chlorine atom in a sample of chlorine gas (group. So far, we. How To Make A Cation Compound.
From www.numerade.com
SOLVED the table below by writing the symbols for the cation How To Make A Cation Compound An ionic compound is never formed between two cations only or two anions only. A proper ionic formula has a cation and an anion in it; Generate a proper name for an ionic compound. In the solid state, ionic compounds are in crystal. Construct a proper formula for an ionic compound. How to make the chemical formulas for a merging. How To Make A Cation Compound.
From www.transtutors.com
(Solved) Complete The Table Below By Writing The Symbols For The How To Make A Cation Compound For example, when each sodium atom in a sample of sodium metal (group 1) gives up one electron to form a sodium cation, na +, and each chlorine atom in a sample of chlorine gas (group. In the solid state, ionic compounds are in crystal. Ionic compounds do not exist as molecules. Recognize polyatomic ions in chemical formulas. If the. How To Make A Cation Compound.
From www.coursehero.com
[Solved] Balancing charges Suppose that X represents an arbitrary How To Make A Cation Compound So far, we have discussed elements and. Ionic compounds do not exist as molecules. How to make the chemical formulas for a merging cation and anion? If the compound contains a cation of a transition metal with two possible charges, then a roman numeral or the corresponding suffix is used to indicate its charge. Recognize polyatomic ions in chemical formulas.. How To Make A Cation Compound.
From www.chegg.com
Solved Complete the table below by writing the symbols for How To Make A Cation Compound How do you write a net ionic equation for the reaction of aqueous. Recognize polyatomic ions in chemical formulas. Ionic compounds do not exist as molecules. Predict the charge of monatomic main group elements based on their. Recognize that metals lose electrons to form cations and that nonmetals gain electrons to form anions. In the solid state, ionic compounds are. How To Make A Cation Compound.
From www.pinterest.com
cation anion compound chemical formulas Writing formulas for ionic How To Make A Cation Compound Recognize polyatomic ions in chemical formulas. Ionic compounds do not exist as molecules. An ionic compound is never formed between two cations only or two anions only. Recognize that metals lose electrons to form cations and that nonmetals gain electrons to form anions. Generate a proper name for an ionic compound. How to make the chemical formulas for a merging. How To Make A Cation Compound.
From www.chegg.com
Solved Complete the table below by writing the symbols for How To Make A Cation Compound A proper ionic formula has a cation and an anion in it; Recognize polyatomic ions in chemical formulas. Ionic compounds do not exist as molecules. Generate a proper name for an ionic compound. Predict the charge of monatomic main group elements based on their. How do you write a net ionic equation for the reaction of aqueous. So far, we. How To Make A Cation Compound.
From www.youtube.com
Make Ionic Compounds from Cations and Anions YouTube How To Make A Cation Compound How do you write a net ionic equation for the reaction of aqueous. Construct a proper formula for an ionic compound. Recognize that metals lose electrons to form cations and that nonmetals gain electrons to form anions. So far, we have discussed elements and. Generate a proper name for an ionic compound. A proper ionic formula has a cation and. How To Make A Cation Compound.
From www.numerade.com
SOLVEDWrite the cation and anion in each compoun… How To Make A Cation Compound How do you write a net ionic equation for the reaction of aqueous. Recognize that metals lose electrons to form cations and that nonmetals gain electrons to form anions. For example, when each sodium atom in a sample of sodium metal (group 1) gives up one electron to form a sodium cation, na +, and each chlorine atom in a. How To Make A Cation Compound.
From brainly.ph
Activity 3 Charges (+) Directions Write the charges (cations and How To Make A Cation Compound A proper ionic formula has a cation and an anion in it; If the compound contains a cation of a transition metal with two possible charges, then a roman numeral or the corresponding suffix is used to indicate its charge. Recognize that metals lose electrons to form cations and that nonmetals gain electrons to form anions. Recognize polyatomic ions in. How To Make A Cation Compound.
From www.thoughtco.com
The Difference Between a Cation and an Anion How To Make A Cation Compound Construct a proper formula for an ionic compound. Predict the charge of monatomic main group elements based on their. Generate a proper name for an ionic compound. A proper ionic formula has a cation and an anion in it; Recognize that metals lose electrons to form cations and that nonmetals gain electrons to form anions. An ionic compound is never. How To Make A Cation Compound.
From karsyntinoconnell.blogspot.com
Cations and Anions List KarsyntinOconnell How To Make A Cation Compound Ionic compounds do not exist as molecules. An ionic compound is never formed between two cations only or two anions only. For example, when each sodium atom in a sample of sodium metal (group 1) gives up one electron to form a sodium cation, na +, and each chlorine atom in a sample of chlorine gas (group. Generate a proper. How To Make A Cation Compound.
From solvedlib.com
Calculate the number of cations and anions in each of… SolvedLib How To Make A Cation Compound In the solid state, ionic compounds are in crystal. Recognize that metals lose electrons to form cations and that nonmetals gain electrons to form anions. Ionic compounds do not exist as molecules. How do you write a net ionic equation for the reaction of aqueous. A proper ionic formula has a cation and an anion in it; For example, when. How To Make A Cation Compound.
From chem.libretexts.org
1.5 Separation of cations in groups Chemistry LibreTexts How To Make A Cation Compound If the compound contains a cation of a transition metal with two possible charges, then a roman numeral or the corresponding suffix is used to indicate its charge. Ionic compounds do not exist as molecules. A proper ionic formula has a cation and an anion in it; Predict the charge of monatomic main group elements based on their. Construct a. How To Make A Cation Compound.
From www.youtube.com
what is an Ion? Cation and Anion Chemistry YouTube How To Make A Cation Compound So far, we have discussed elements and. An ionic compound is never formed between two cations only or two anions only. How to make the chemical formulas for a merging cation and anion? How do you write a net ionic equation for the reaction of aqueous. Recognize that metals lose electrons to form cations and that nonmetals gain electrons to. How To Make A Cation Compound.
From www.chegg.com
Solved ionic compound cation anion anion cation Na Naci СІ How To Make A Cation Compound If the compound contains a cation of a transition metal with two possible charges, then a roman numeral or the corresponding suffix is used to indicate its charge. Generate a proper name for an ionic compound. Predict the charge of monatomic main group elements based on their. A proper ionic formula has a cation and an anion in it; How. How To Make A Cation Compound.
From www.numerade.com
the table below by writing the symbols for the cation How To Make A Cation Compound Generate a proper name for an ionic compound. How to make the chemical formulas for a merging cation and anion? Construct a proper formula for an ionic compound. In the solid state, ionic compounds are in crystal. How do you write a net ionic equation for the reaction of aqueous. Recognize polyatomic ions in chemical formulas. Predict the charge of. How To Make A Cation Compound.
From www.numerade.com
Complete the table by filling in the formula for the ionic compound How To Make A Cation Compound For example, when each sodium atom in a sample of sodium metal (group 1) gives up one electron to form a sodium cation, na +, and each chlorine atom in a sample of chlorine gas (group. Generate a proper name for an ionic compound. If the compound contains a cation of a transition metal with two possible charges, then a. How To Make A Cation Compound.
From www.youtube.com
COMMON CATIONS AND ANIONS YouTube How To Make A Cation Compound Generate a proper name for an ionic compound. Recognize polyatomic ions in chemical formulas. How to make the chemical formulas for a merging cation and anion? Construct a proper formula for an ionic compound. For example, when each sodium atom in a sample of sodium metal (group 1) gives up one electron to form a sodium cation, na +, and. How To Make A Cation Compound.
From general.chemistrysteps.com
Writing Chemical Formulas For Ionic Compounds Chemistry Steps How To Make A Cation Compound In the solid state, ionic compounds are in crystal. How do you write a net ionic equation for the reaction of aqueous. If the compound contains a cation of a transition metal with two possible charges, then a roman numeral or the corresponding suffix is used to indicate its charge. Ionic compounds do not exist as molecules. Recognize that metals. How To Make A Cation Compound.
From brainly.com
Complete the table below by writing the symbols for the cation and How To Make A Cation Compound How to make the chemical formulas for a merging cation and anion? How do you write a net ionic equation for the reaction of aqueous. If the compound contains a cation of a transition metal with two possible charges, then a roman numeral or the corresponding suffix is used to indicate its charge. In the solid state, ionic compounds are. How To Make A Cation Compound.
From www.transtutors.com
(Solved) Complete The Table Below By Writing The Symbols For The How To Make A Cation Compound Generate a proper name for an ionic compound. A proper ionic formula has a cation and an anion in it; How do you write a net ionic equation for the reaction of aqueous. An ionic compound is never formed between two cations only or two anions only. In the solid state, ionic compounds are in crystal. For example, when each. How To Make A Cation Compound.
From brainly.in
Write the formula of the compounds from the given cation and anions How To Make A Cation Compound Generate a proper name for an ionic compound. Predict the charge of monatomic main group elements based on their. So far, we have discussed elements and. How to make the chemical formulas for a merging cation and anion? In the solid state, ionic compounds are in crystal. A proper ionic formula has a cation and an anion in it; How. How To Make A Cation Compound.
From animalia-life.club
Cation And Anion How To Make A Cation Compound A proper ionic formula has a cation and an anion in it; Recognize polyatomic ions in chemical formulas. So far, we have discussed elements and. Ionic compounds do not exist as molecules. For example, when each sodium atom in a sample of sodium metal (group 1) gives up one electron to form a sodium cation, na +, and each chlorine. How To Make A Cation Compound.
From study.com
Cation Definition, Formation & Examples Lesson How To Make A Cation Compound Ionic compounds do not exist as molecules. Generate a proper name for an ionic compound. A proper ionic formula has a cation and an anion in it; So far, we have discussed elements and. How do you write a net ionic equation for the reaction of aqueous. Recognize polyatomic ions in chemical formulas. How to make the chemical formulas for. How To Make A Cation Compound.
From www.bartleby.com
Answered Complete the table below by writing the… bartleby How To Make A Cation Compound For example, when each sodium atom in a sample of sodium metal (group 1) gives up one electron to form a sodium cation, na +, and each chlorine atom in a sample of chlorine gas (group. Recognize polyatomic ions in chemical formulas. If the compound contains a cation of a transition metal with two possible charges, then a roman numeral. How To Make A Cation Compound.
From chem.libretexts.org
3.3 Naming Ionic Compounds Chemistry LibreTexts How To Make A Cation Compound How to make the chemical formulas for a merging cation and anion? Recognize that metals lose electrons to form cations and that nonmetals gain electrons to form anions. For example, when each sodium atom in a sample of sodium metal (group 1) gives up one electron to form a sodium cation, na +, and each chlorine atom in a sample. How To Make A Cation Compound.
From www.numerade.com
SOLVED Question 11 When meta and nonmeta exchange electrons t0 make How To Make A Cation Compound Ionic compounds do not exist as molecules. Recognize polyatomic ions in chemical formulas. Generate a proper name for an ionic compound. How do you write a net ionic equation for the reaction of aqueous. Construct a proper formula for an ionic compound. Recognize that metals lose electrons to form cations and that nonmetals gain electrons to form anions. Predict the. How To Make A Cation Compound.
From ar.inspiredpencil.com
List Of Cations How To Make A Cation Compound An ionic compound is never formed between two cations only or two anions only. A proper ionic formula has a cation and an anion in it; How to make the chemical formulas for a merging cation and anion? In the solid state, ionic compounds are in crystal. If the compound contains a cation of a transition metal with two possible. How To Make A Cation Compound.
From aboveknowledge.blogspot.com
How to remember the charge of cation and anion How To Make A Cation Compound Generate a proper name for an ionic compound. Construct a proper formula for an ionic compound. Recognize polyatomic ions in chemical formulas. A proper ionic formula has a cation and an anion in it; Recognize that metals lose electrons to form cations and that nonmetals gain electrons to form anions. If the compound contains a cation of a transition metal. How To Make A Cation Compound.
From www.chegg.com
Solved Table 4, Ionic Compounds and their Names Formula of How To Make A Cation Compound If the compound contains a cation of a transition metal with two possible charges, then a roman numeral or the corresponding suffix is used to indicate its charge. An ionic compound is never formed between two cations only or two anions only. Predict the charge of monatomic main group elements based on their. Construct a proper formula for an ionic. How To Make A Cation Compound.
From www.youtube.com
How to identify cations and anions in ionic compounds. YouTube How To Make A Cation Compound If the compound contains a cation of a transition metal with two possible charges, then a roman numeral or the corresponding suffix is used to indicate its charge. In the solid state, ionic compounds are in crystal. Generate a proper name for an ionic compound. Recognize that metals lose electrons to form cations and that nonmetals gain electrons to form. How To Make A Cation Compound.
From www.chegg.com
Solved Complete the table below by writing the symbols for How To Make A Cation Compound In the solid state, ionic compounds are in crystal. Recognize polyatomic ions in chemical formulas. How do you write a net ionic equation for the reaction of aqueous. So far, we have discussed elements and. For example, when each sodium atom in a sample of sodium metal (group 1) gives up one electron to form a sodium cation, na +,. How To Make A Cation Compound.