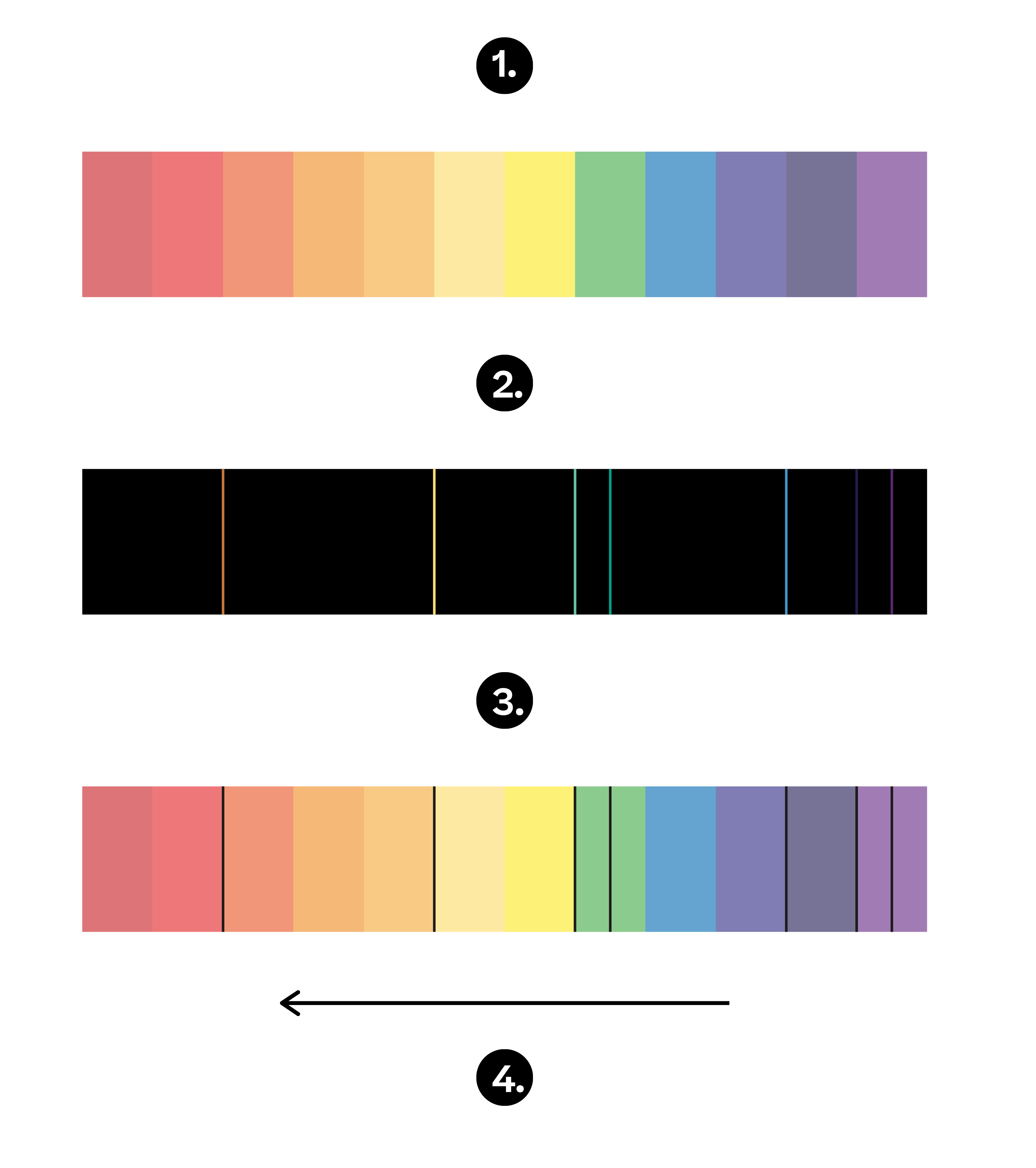
Spectra Emission Density . You will be able to distinguish between emission and absorption lines in a spectrum. The probability/s that a molecule in state 2 exposed to radiation of spectral density ρ(ν) will emit a quantum hν and pass to state 1. Only emits light at particular wavelengths, giving. You will know how spectral lines. A low density, cool gas in front of a hotter source of a continuous. When hydrogen gas is placed. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. Every element has a unique set of absorption and emission lines, or. The emission spectrum of a chemical element or chemical compound is the spectrum of frequencies of electromagnetic radiation. A low density, hot gas seen against a cooler background emits a bright line or emission line spectrum. Simplified illustration of absorption and emission spectra.
from evulpo.com
You will know how spectral lines. The probability/s that a molecule in state 2 exposed to radiation of spectral density ρ(ν) will emit a quantum hν and pass to state 1. Simplified illustration of absorption and emission spectra. Every element has a unique set of absorption and emission lines, or. A low density, cool gas in front of a hotter source of a continuous. The emission spectrum of a chemical element or chemical compound is the spectrum of frequencies of electromagnetic radiation. A low density, hot gas seen against a cooler background emits a bright line or emission line spectrum. You will be able to distinguish between emission and absorption lines in a spectrum. When hydrogen gas is placed. Only emits light at particular wavelengths, giving.
Energy levels and spectra Physics Explanation & Exercises evulpo
Spectra Emission Density The probability/s that a molecule in state 2 exposed to radiation of spectral density ρ(ν) will emit a quantum hν and pass to state 1. A low density, cool gas in front of a hotter source of a continuous. You will be able to distinguish between emission and absorption lines in a spectrum. When hydrogen gas is placed. The probability/s that a molecule in state 2 exposed to radiation of spectral density ρ(ν) will emit a quantum hν and pass to state 1. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. Only emits light at particular wavelengths, giving. The emission spectrum of a chemical element or chemical compound is the spectrum of frequencies of electromagnetic radiation. Every element has a unique set of absorption and emission lines, or. A low density, hot gas seen against a cooler background emits a bright line or emission line spectrum. You will know how spectral lines. Simplified illustration of absorption and emission spectra.
From www.researchgate.net
Emission spectra of the used lightcuring units. Download Scientific Spectra Emission Density The probability/s that a molecule in state 2 exposed to radiation of spectral density ρ(ν) will emit a quantum hν and pass to state 1. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. Only emits light at particular wavelengths, giving. You will. Spectra Emission Density.
From courses.lumenlearning.com
The Spectrum Astronomy Spectra Emission Density A low density, hot gas seen against a cooler background emits a bright line or emission line spectrum. Simplified illustration of absorption and emission spectra. The probability/s that a molecule in state 2 exposed to radiation of spectral density ρ(ν) will emit a quantum hν and pass to state 1. Only emits light at particular wavelengths, giving. You will be. Spectra Emission Density.
From users.highland.edu
Atomic Spectra and Models of the Atom Spectra Emission Density Every element has a unique set of absorption and emission lines, or. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. The probability/s that a molecule in state 2 exposed to radiation of spectral density ρ(ν) will emit a quantum hν and pass. Spectra Emission Density.
From www.researchgate.net
Emission intensity spectrum for random lasers containing Rh6G and Al 2 Spectra Emission Density Only emits light at particular wavelengths, giving. You will know how spectral lines. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. You will be able to distinguish between emission and absorption lines in a spectrum. The emission spectrum of a chemical element. Spectra Emission Density.
From www.coursehero.com
Spectroscopy in Astronomy Astronomy Course Hero Spectra Emission Density A low density, cool gas in front of a hotter source of a continuous. Simplified illustration of absorption and emission spectra. A low density, hot gas seen against a cooler background emits a bright line or emission line spectrum. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is. Spectra Emission Density.
From www.heraeus.com
Emission spectra of infrared lamps Spectra Emission Density Every element has a unique set of absorption and emission lines, or. You will know how spectral lines. You will be able to distinguish between emission and absorption lines in a spectrum. A low density, cool gas in front of a hotter source of a continuous. The probability/s that a molecule in state 2 exposed to radiation of spectral density. Spectra Emission Density.
From www.slideserve.com
PPT Absorption / Emission of Photons and Conservation of Energy Spectra Emission Density Simplified illustration of absorption and emission spectra. When hydrogen gas is placed. The probability/s that a molecule in state 2 exposed to radiation of spectral density ρ(ν) will emit a quantum hν and pass to state 1. You will be able to distinguish between emission and absorption lines in a spectrum. The emission spectrum of a chemical element or chemical. Spectra Emission Density.
From www.espacotempo.com.br
O que é a classificação espectral das estrelas e pra que ela serve Spectra Emission Density A low density, cool gas in front of a hotter source of a continuous. You will be able to distinguish between emission and absorption lines in a spectrum. Only emits light at particular wavelengths, giving. A low density, hot gas seen against a cooler background emits a bright line or emission line spectrum. The probability/s that a molecule in state. Spectra Emission Density.
From web.njit.edu
Radio Astronomy Lecture Number 10 Spectra Emission Density The emission spectrum of a chemical element or chemical compound is the spectrum of frequencies of electromagnetic radiation. You will be able to distinguish between emission and absorption lines in a spectrum. When hydrogen gas is placed. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to. Spectra Emission Density.
From www.researchgate.net
(color online). Main panel spectral density of the photon emission Spectra Emission Density Simplified illustration of absorption and emission spectra. A low density, hot gas seen against a cooler background emits a bright line or emission line spectrum. You will be able to distinguish between emission and absorption lines in a spectrum. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is. Spectra Emission Density.
From laderarctic.weebly.com
Emission spectra laderarctic Spectra Emission Density A low density, hot gas seen against a cooler background emits a bright line or emission line spectrum. Every element has a unique set of absorption and emission lines, or. The emission spectrum of a chemical element or chemical compound is the spectrum of frequencies of electromagnetic radiation. You will know how spectral lines. Only emits light at particular wavelengths,. Spectra Emission Density.
From scisyn.com
Spectra Examples Spectra Emission Density The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. A low density, hot gas seen against a cooler background emits a bright line or emission line spectrum. Simplified illustration of absorption and emission spectra. When hydrogen gas is placed. A low density, cool. Spectra Emission Density.
From www.researchgate.net
The emission spectra from He I's 3p 1 P 0 level density are shown for Spectra Emission Density The emission spectrum of a chemical element or chemical compound is the spectrum of frequencies of electromagnetic radiation. When hydrogen gas is placed. Every element has a unique set of absorption and emission lines, or. A low density, hot gas seen against a cooler background emits a bright line or emission line spectrum. You will be able to distinguish between. Spectra Emission Density.
From www.researchgate.net
Typical PL excitation spectra and emission spectra for... Download Spectra Emission Density A low density, hot gas seen against a cooler background emits a bright line or emission line spectrum. Simplified illustration of absorption and emission spectra. You will know how spectral lines. You will be able to distinguish between emission and absorption lines in a spectrum. Only emits light at particular wavelengths, giving. When hydrogen gas is placed. The probability/s that. Spectra Emission Density.
From www.researchgate.net
(a)(d) Emission spectra of four diamond specimens grown by various Spectra Emission Density A low density, cool gas in front of a hotter source of a continuous. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. You will know how spectral lines. When hydrogen gas is placed. Simplified illustration of absorption and emission spectra. You will. Spectra Emission Density.
From ppt-online.org
Emission spectrum of H презентация онлайн Spectra Emission Density When hydrogen gas is placed. You will be able to distinguish between emission and absorption lines in a spectrum. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. A low density, hot gas seen against a cooler background emits a bright line or. Spectra Emission Density.
From www.astronomy.ohio-state.edu
Lecture 24 Matter & Light Spectra Emission Density The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. A low density, hot gas seen against a cooler background emits a bright line or emission line spectrum. Every element has a unique set of absorption and emission lines, or. Only emits light at. Spectra Emission Density.
From www.e-education.psu.edu
The "Greenhouse Effect," and Global Warming METEO 3 Introductory Spectra Emission Density A low density, cool gas in front of a hotter source of a continuous. The emission spectrum of a chemical element or chemical compound is the spectrum of frequencies of electromagnetic radiation. The probability/s that a molecule in state 2 exposed to radiation of spectral density ρ(ν) will emit a quantum hν and pass to state 1. You will be. Spectra Emission Density.
From www.researchgate.net
Emission spectra from lowdensity target at 30 Gd concentration (solid Spectra Emission Density Every element has a unique set of absorption and emission lines, or. Simplified illustration of absorption and emission spectra. You will be able to distinguish between emission and absorption lines in a spectrum. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. A. Spectra Emission Density.
From poozacreations.blogspot.com
Types of emission and absorption spectra Pooza Creations Spectra Emission Density Simplified illustration of absorption and emission spectra. When hydrogen gas is placed. The emission spectrum of a chemical element or chemical compound is the spectrum of frequencies of electromagnetic radiation. You will know how spectral lines. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat. Spectra Emission Density.
From courses.lumenlearning.com
Energy Chemistry I Spectra Emission Density Only emits light at particular wavelengths, giving. The probability/s that a molecule in state 2 exposed to radiation of spectral density ρ(ν) will emit a quantum hν and pass to state 1. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. Every element. Spectra Emission Density.
From studyposter.blogspot.com
How Is A Stars Emission Spectrum Used To Study Stars Study Poster Spectra Emission Density The emission spectrum of a chemical element or chemical compound is the spectrum of frequencies of electromagnetic radiation. When hydrogen gas is placed. A low density, hot gas seen against a cooler background emits a bright line or emission line spectrum. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the. Spectra Emission Density.
From brilliant.org
Spectral Properties of Sunlight Practice Problems Online Brilliant Spectra Emission Density Simplified illustration of absorption and emission spectra. The emission spectrum of a chemical element or chemical compound is the spectrum of frequencies of electromagnetic radiation. The probability/s that a molecule in state 2 exposed to radiation of spectral density ρ(ν) will emit a quantum hν and pass to state 1. You will be able to distinguish between emission and absorption. Spectra Emission Density.
From www.researchgate.net
Optical emission spectra from hydroxyl radical OH species in the Spectra Emission Density Only emits light at particular wavelengths, giving. You will be able to distinguish between emission and absorption lines in a spectrum. A low density, hot gas seen against a cooler background emits a bright line or emission line spectrum. A low density, cool gas in front of a hotter source of a continuous. Every element has a unique set of. Spectra Emission Density.
From www.sun.org
spectrum Spectra Emission Density You will be able to distinguish between emission and absorption lines in a spectrum. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. The emission spectrum of a chemical element or chemical compound is the spectrum of frequencies of electromagnetic radiation. The probability/s. Spectra Emission Density.
From www.researchgate.net
(a) Typical emission density spectrum for airglow and aurora. (b Spectra Emission Density When hydrogen gas is placed. You will know how spectral lines. A low density, hot gas seen against a cooler background emits a bright line or emission line spectrum. Only emits light at particular wavelengths, giving. You will be able to distinguish between emission and absorption lines in a spectrum. The emission spectrum of a chemical element or chemical compound. Spectra Emission Density.
From www.researchgate.net
Simulated spectra of sulfur emission from a thin target at a density of Spectra Emission Density A low density, cool gas in front of a hotter source of a continuous. Every element has a unique set of absorption and emission lines, or. The probability/s that a molecule in state 2 exposed to radiation of spectral density ρ(ν) will emit a quantum hν and pass to state 1. Simplified illustration of absorption and emission spectra. You will. Spectra Emission Density.
From webbtelescope.org
Spectroscopy 101 Types of Spectra and Spectroscopy b Spectra Emission Density A low density, cool gas in front of a hotter source of a continuous. Simplified illustration of absorption and emission spectra. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. Every element has a unique set of absorption and emission lines, or. A. Spectra Emission Density.
From www.researchgate.net
a The PL spectra as a function of the excitation power density, b the Spectra Emission Density Only emits light at particular wavelengths, giving. Every element has a unique set of absorption and emission lines, or. Simplified illustration of absorption and emission spectra. A low density, hot gas seen against a cooler background emits a bright line or emission line spectrum. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light. Spectra Emission Density.
From evulpo.com
Energy levels and spectra Physics Explanation & Exercises evulpo Spectra Emission Density The probability/s that a molecule in state 2 exposed to radiation of spectral density ρ(ν) will emit a quantum hν and pass to state 1. Every element has a unique set of absorption and emission lines, or. Simplified illustration of absorption and emission spectra. You will be able to distinguish between emission and absorption lines in a spectrum. A low. Spectra Emission Density.
From www.cv.nrao.edu
7 Spectral Lines‣ Essential Radio Astronomy Spectra Emission Density You will be able to distinguish between emission and absorption lines in a spectrum. A low density, cool gas in front of a hotter source of a continuous. The emission spectrum of a chemical element or chemical compound is the spectrum of frequencies of electromagnetic radiation. When hydrogen gas is placed. The probability/s that a molecule in state 2 exposed. Spectra Emission Density.
From phys.libretexts.org
2.6 Lines Spectra Emission and Absorption Lines Physics LibreTexts Spectra Emission Density Every element has a unique set of absorption and emission lines, or. A low density, cool gas in front of a hotter source of a continuous. Simplified illustration of absorption and emission spectra. The probability/s that a molecule in state 2 exposed to radiation of spectral density ρ(ν) will emit a quantum hν and pass to state 1. You will. Spectra Emission Density.
From www.researchgate.net
Optical emission spectra of O2, N2, Ar, and Air feeding gases plasma Spectra Emission Density You will be able to distinguish between emission and absorption lines in a spectrum. Only emits light at particular wavelengths, giving. A low density, hot gas seen against a cooler background emits a bright line or emission line spectrum. When hydrogen gas is placed. Every element has a unique set of absorption and emission lines, or. A low density, cool. Spectra Emission Density.
From www.astronomynotes.com
Radiation Spectra Emission Density The probability/s that a molecule in state 2 exposed to radiation of spectral density ρ(ν) will emit a quantum hν and pass to state 1. You will be able to distinguish between emission and absorption lines in a spectrum. When hydrogen gas is placed. A low density, cool gas in front of a hotter source of a continuous. Every element. Spectra Emission Density.
From www.researchgate.net
The fluorescence spectra of aqueous solutions of ThT at different Spectra Emission Density Every element has a unique set of absorption and emission lines, or. A low density, hot gas seen against a cooler background emits a bright line or emission line spectrum. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. Simplified illustration of absorption. Spectra Emission Density.