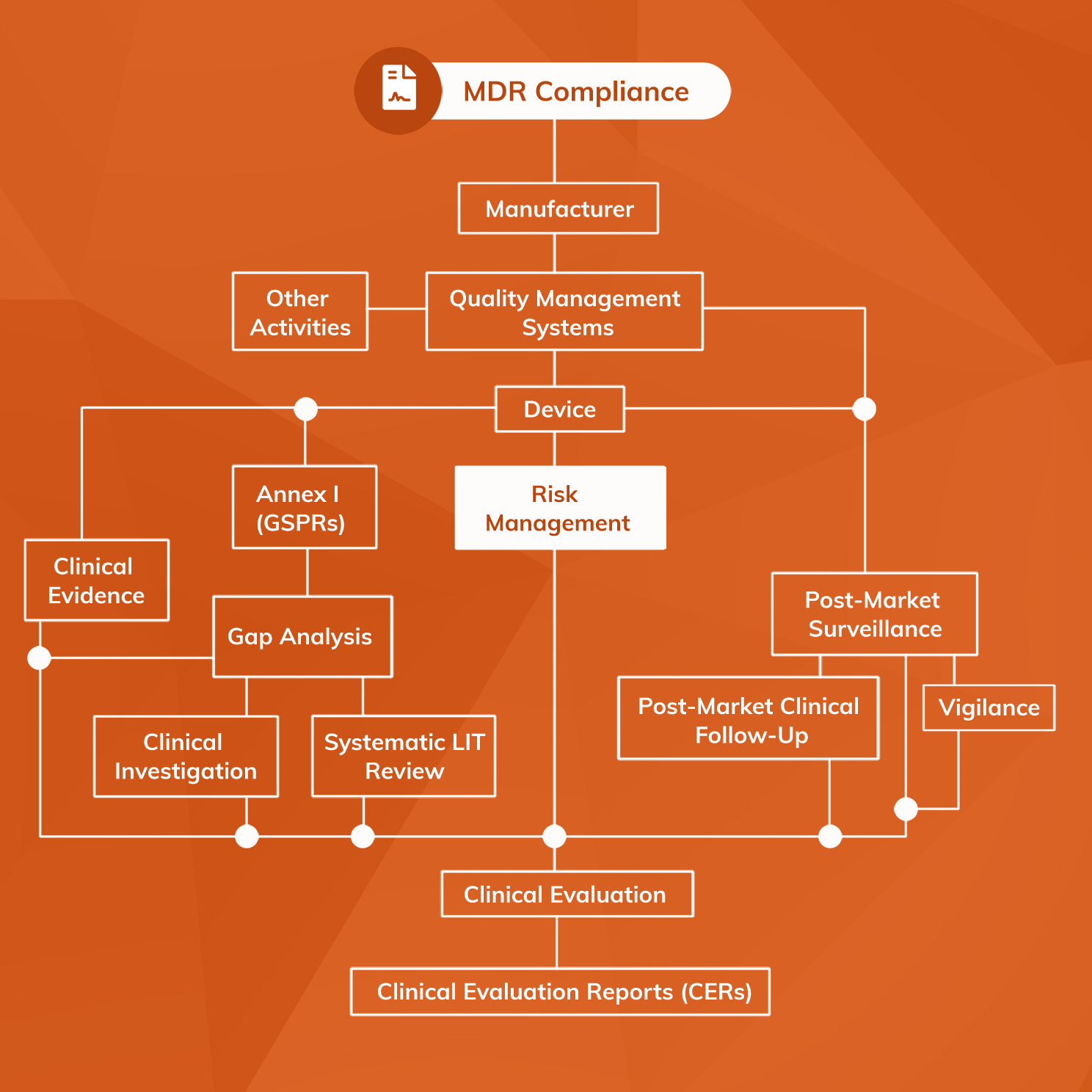
Medical Device Risk Management File . this document specifies terminology, principles and a process for risk management of medical devices,. international standard bs en iso 14971 [1] was developed to provide a process to assist manufacturers in identifying the hazards. When implementing a quality system to: this document specifies terminology, principles and a process for risk management of medical devices,. Are you sure you’re organizing and tracking these files in. Conduct risk analysis, where appropriate,. introduction to the definitive guide to iso 14971 risk management for medical devices. when to use risk management activities.
from www.mantrasystems.co.uk
this document specifies terminology, principles and a process for risk management of medical devices,. Are you sure you’re organizing and tracking these files in. introduction to the definitive guide to iso 14971 risk management for medical devices. international standard bs en iso 14971 [1] was developed to provide a process to assist manufacturers in identifying the hazards. this document specifies terminology, principles and a process for risk management of medical devices,. when to use risk management activities. Conduct risk analysis, where appropriate,. When implementing a quality system to:
Medical Device Risk Management, Assessment and Analysis
Medical Device Risk Management File this document specifies terminology, principles and a process for risk management of medical devices,. when to use risk management activities. international standard bs en iso 14971 [1] was developed to provide a process to assist manufacturers in identifying the hazards. Conduct risk analysis, where appropriate,. this document specifies terminology, principles and a process for risk management of medical devices,. introduction to the definitive guide to iso 14971 risk management for medical devices. When implementing a quality system to: this document specifies terminology, principles and a process for risk management of medical devices,. Are you sure you’re organizing and tracking these files in.
From www.orielstat.com
Preparing a Medical Device Risk Management Review and Report Medical Device Risk Management File this document specifies terminology, principles and a process for risk management of medical devices,. Conduct risk analysis, where appropriate,. When implementing a quality system to: Are you sure you’re organizing and tracking these files in. international standard bs en iso 14971 [1] was developed to provide a process to assist manufacturers in identifying the hazards. introduction to. Medical Device Risk Management File.
From tsqasia.com
ISO 14971 Quick Review of Medical Devices' Risk Management Standard Medical Device Risk Management File Are you sure you’re organizing and tracking these files in. international standard bs en iso 14971 [1] was developed to provide a process to assist manufacturers in identifying the hazards. introduction to the definitive guide to iso 14971 risk management for medical devices. When implementing a quality system to: when to use risk management activities. this. Medical Device Risk Management File.
From www.presentationeze.com
Medical Device Risk Management Information & TrainingPresentationEZE Medical Device Risk Management File this document specifies terminology, principles and a process for risk management of medical devices,. Are you sure you’re organizing and tracking these files in. Conduct risk analysis, where appropriate,. introduction to the definitive guide to iso 14971 risk management for medical devices. When implementing a quality system to: international standard bs en iso 14971 [1] was developed. Medical Device Risk Management File.
From www.greenlight.guru
Understanding ISO 14971 Medical Device Risk Management Medical Device Risk Management File Are you sure you’re organizing and tracking these files in. When implementing a quality system to: introduction to the definitive guide to iso 14971 risk management for medical devices. this document specifies terminology, principles and a process for risk management of medical devices,. Conduct risk analysis, where appropriate,. when to use risk management activities. this document. Medical Device Risk Management File.
From medicaldevicehq.com
How to integrate proactive safety by design Medical Device HQ Medical Device Risk Management File when to use risk management activities. Are you sure you’re organizing and tracking these files in. this document specifies terminology, principles and a process for risk management of medical devices,. this document specifies terminology, principles and a process for risk management of medical devices,. international standard bs en iso 14971 [1] was developed to provide a. Medical Device Risk Management File.
From medicaldevicehq.com
How to work with medical device risk management Medical Device HQ Medical Device Risk Management File introduction to the definitive guide to iso 14971 risk management for medical devices. this document specifies terminology, principles and a process for risk management of medical devices,. When implementing a quality system to: this document specifies terminology, principles and a process for risk management of medical devices,. Are you sure you’re organizing and tracking these files in.. Medical Device Risk Management File.
From www.perforce.com
Condensed Guide to Medical Device Requirements Management Perforce Medical Device Risk Management File when to use risk management activities. When implementing a quality system to: this document specifies terminology, principles and a process for risk management of medical devices,. this document specifies terminology, principles and a process for risk management of medical devices,. Are you sure you’re organizing and tracking these files in. introduction to the definitive guide to. Medical Device Risk Management File.
From www.qualio.com
ISO 13485 risk management plan template Medical Device Risk Management File international standard bs en iso 14971 [1] was developed to provide a process to assist manufacturers in identifying the hazards. this document specifies terminology, principles and a process for risk management of medical devices,. Conduct risk analysis, where appropriate,. Are you sure you’re organizing and tracking these files in. When implementing a quality system to: when to. Medical Device Risk Management File.
From mariahuble1966.blogspot.com
Iso14971 Risk Management Template / Risk Management Procedure Medical Device Risk Management File this document specifies terminology, principles and a process for risk management of medical devices,. international standard bs en iso 14971 [1] was developed to provide a process to assist manufacturers in identifying the hazards. when to use risk management activities. Are you sure you’re organizing and tracking these files in. When implementing a quality system to: Conduct. Medical Device Risk Management File.
From www.orielstat.com
ISO 149712019 Basics of Medical Device Risk Management Medical Device Risk Management File this document specifies terminology, principles and a process for risk management of medical devices,. when to use risk management activities. international standard bs en iso 14971 [1] was developed to provide a process to assist manufacturers in identifying the hazards. Conduct risk analysis, where appropriate,. introduction to the definitive guide to iso 14971 risk management for. Medical Device Risk Management File.
From sunstonepilot.com
The Big Picture for Medical Device Risk Management Sunstone Pilot, Inc. Medical Device Risk Management File international standard bs en iso 14971 [1] was developed to provide a process to assist manufacturers in identifying the hazards. this document specifies terminology, principles and a process for risk management of medical devices,. Conduct risk analysis, where appropriate,. introduction to the definitive guide to iso 14971 risk management for medical devices. when to use risk. Medical Device Risk Management File.
From www.orielstat.com
Choosing the right medical device risk management tools Medical Device Risk Management File Conduct risk analysis, where appropriate,. international standard bs en iso 14971 [1] was developed to provide a process to assist manufacturers in identifying the hazards. introduction to the definitive guide to iso 14971 risk management for medical devices. Are you sure you’re organizing and tracking these files in. when to use risk management activities. When implementing a. Medical Device Risk Management File.
From kvalito.ch
Risk Management for Medical Devices ISO 149712019 Kvalito Medical Device Risk Management File this document specifies terminology, principles and a process for risk management of medical devices,. Are you sure you’re organizing and tracking these files in. When implementing a quality system to: when to use risk management activities. Conduct risk analysis, where appropriate,. introduction to the definitive guide to iso 14971 risk management for medical devices. this document. Medical Device Risk Management File.
From www.viralcovert.com
Risk Management Plan Template (medical Device And Iso 14971) Template Medical Device Risk Management File this document specifies terminology, principles and a process for risk management of medical devices,. Are you sure you’re organizing and tracking these files in. When implementing a quality system to: introduction to the definitive guide to iso 14971 risk management for medical devices. Conduct risk analysis, where appropriate,. this document specifies terminology, principles and a process for. Medical Device Risk Management File.
From www.researchgate.net
(PDF) ISO 14971Medical Device Risk Management Standard Medical Device Risk Management File introduction to the definitive guide to iso 14971 risk management for medical devices. Conduct risk analysis, where appropriate,. When implementing a quality system to: when to use risk management activities. this document specifies terminology, principles and a process for risk management of medical devices,. international standard bs en iso 14971 [1] was developed to provide a. Medical Device Risk Management File.
From www.vrogue.co
Choosing The Right Medical Device Risk Management Too vrogue.co Medical Device Risk Management File Are you sure you’re organizing and tracking these files in. introduction to the definitive guide to iso 14971 risk management for medical devices. this document specifies terminology, principles and a process for risk management of medical devices,. international standard bs en iso 14971 [1] was developed to provide a process to assist manufacturers in identifying the hazards.. Medical Device Risk Management File.
From www.mpo-mag.com
EMC Risk Management Files For Medical Device Developers Medical Medical Device Risk Management File when to use risk management activities. Are you sure you’re organizing and tracking these files in. Conduct risk analysis, where appropriate,. international standard bs en iso 14971 [1] was developed to provide a process to assist manufacturers in identifying the hazards. this document specifies terminology, principles and a process for risk management of medical devices,. introduction. Medical Device Risk Management File.
From www.joharidigital.com
Risk Management Process for Medical Devices Medical Device Johari Medical Device Risk Management File this document specifies terminology, principles and a process for risk management of medical devices,. Are you sure you’re organizing and tracking these files in. When implementing a quality system to: introduction to the definitive guide to iso 14971 risk management for medical devices. when to use risk management activities. this document specifies terminology, principles and a. Medical Device Risk Management File.
From www.greenlight.guru
ISO 14971 Risk Management Definitive Guide for Medical Devices & PDF Medical Device Risk Management File When implementing a quality system to: Conduct risk analysis, where appropriate,. when to use risk management activities. Are you sure you’re organizing and tracking these files in. this document specifies terminology, principles and a process for risk management of medical devices,. introduction to the definitive guide to iso 14971 risk management for medical devices. international standard. Medical Device Risk Management File.
From www.orielstat.com
Creating a Medical Device Risk Management Plan and Doing Analysis Medical Device Risk Management File this document specifies terminology, principles and a process for risk management of medical devices,. this document specifies terminology, principles and a process for risk management of medical devices,. when to use risk management activities. Conduct risk analysis, where appropriate,. international standard bs en iso 14971 [1] was developed to provide a process to assist manufacturers in. Medical Device Risk Management File.
From www.mrcomp.com
Risk Management GmbH Medical Device Risk Management File this document specifies terminology, principles and a process for risk management of medical devices,. when to use risk management activities. international standard bs en iso 14971 [1] was developed to provide a process to assist manufacturers in identifying the hazards. Conduct risk analysis, where appropriate,. this document specifies terminology, principles and a process for risk management. Medical Device Risk Management File.
From www.mpo-mag.com
Areas To Consider In Medical Device Risk Management Medical Product Medical Device Risk Management File this document specifies terminology, principles and a process for risk management of medical devices,. When implementing a quality system to: Conduct risk analysis, where appropriate,. when to use risk management activities. international standard bs en iso 14971 [1] was developed to provide a process to assist manufacturers in identifying the hazards. Are you sure you’re organizing and. Medical Device Risk Management File.
From allimagesbooster.blogspot.com
Iso14971 Risk Management Template Iso 14971 2019 Changes In The Medical Device Risk Management File this document specifies terminology, principles and a process for risk management of medical devices,. this document specifies terminology, principles and a process for risk management of medical devices,. international standard bs en iso 14971 [1] was developed to provide a process to assist manufacturers in identifying the hazards. when to use risk management activities. Are you. Medical Device Risk Management File.
From shotnelo.weebly.com
Medical device risk assessment template shotnelo Medical Device Risk Management File international standard bs en iso 14971 [1] was developed to provide a process to assist manufacturers in identifying the hazards. Conduct risk analysis, where appropriate,. this document specifies terminology, principles and a process for risk management of medical devices,. this document specifies terminology, principles and a process for risk management of medical devices,. When implementing a quality. Medical Device Risk Management File.
From www.meddeviceonline.com
Managing Risk For Medical Device Clinical Trials Medical Device Risk Management File this document specifies terminology, principles and a process for risk management of medical devices,. Conduct risk analysis, where appropriate,. when to use risk management activities. international standard bs en iso 14971 [1] was developed to provide a process to assist manufacturers in identifying the hazards. When implementing a quality system to: Are you sure you’re organizing and. Medical Device Risk Management File.
From www.mindflowdesign.com
How to Start a Medical Device Risk Management Plan Medical Device Risk Management File Conduct risk analysis, where appropriate,. introduction to the definitive guide to iso 14971 risk management for medical devices. this document specifies terminology, principles and a process for risk management of medical devices,. international standard bs en iso 14971 [1] was developed to provide a process to assist manufacturers in identifying the hazards. this document specifies terminology,. Medical Device Risk Management File.
From www.mantrasystems.co.uk
Medical Device Risk Management, Assessment and Analysis Medical Device Risk Management File when to use risk management activities. When implementing a quality system to: international standard bs en iso 14971 [1] was developed to provide a process to assist manufacturers in identifying the hazards. Are you sure you’re organizing and tracking these files in. introduction to the definitive guide to iso 14971 risk management for medical devices. this. Medical Device Risk Management File.
From medicaldevicehq.com
Using MS Excel for medical device risk management Medical Device Risk Management File introduction to the definitive guide to iso 14971 risk management for medical devices. Conduct risk analysis, where appropriate,. international standard bs en iso 14971 [1] was developed to provide a process to assist manufacturers in identifying the hazards. When implementing a quality system to: when to use risk management activities. Are you sure you’re organizing and tracking. Medical Device Risk Management File.
From soulcompas.com
Iso 14971 Risk Management Plan Template Medical Device Risk Management File this document specifies terminology, principles and a process for risk management of medical devices,. when to use risk management activities. introduction to the definitive guide to iso 14971 risk management for medical devices. Conduct risk analysis, where appropriate,. this document specifies terminology, principles and a process for risk management of medical devices,. international standard bs. Medical Device Risk Management File.
From www.meddeviceonline.com
Managing Risk For Medical Device Clinical Trials Medical Device Risk Management File this document specifies terminology, principles and a process for risk management of medical devices,. Are you sure you’re organizing and tracking these files in. When implementing a quality system to: introduction to the definitive guide to iso 14971 risk management for medical devices. when to use risk management activities. this document specifies terminology, principles and a. Medical Device Risk Management File.
From sunstonepilot.com
The Big Picture for Medical Device Risk Management Sunstone Pilot, Inc. Medical Device Risk Management File international standard bs en iso 14971 [1] was developed to provide a process to assist manufacturers in identifying the hazards. introduction to the definitive guide to iso 14971 risk management for medical devices. this document specifies terminology, principles and a process for risk management of medical devices,. Conduct risk analysis, where appropriate,. when to use risk. Medical Device Risk Management File.
From omcmedical.com
Risk Management of medical devices under MDR OMC Medical Medical Device Risk Management File this document specifies terminology, principles and a process for risk management of medical devices,. international standard bs en iso 14971 [1] was developed to provide a process to assist manufacturers in identifying the hazards. When implementing a quality system to: Conduct risk analysis, where appropriate,. Are you sure you’re organizing and tracking these files in. this document. Medical Device Risk Management File.
From www.achievexl.com
Risk Management File Structure Made Easy for ISO 14971 Compliance Medical Device Risk Management File When implementing a quality system to: this document specifies terminology, principles and a process for risk management of medical devices,. when to use risk management activities. Are you sure you’re organizing and tracking these files in. Conduct risk analysis, where appropriate,. international standard bs en iso 14971 [1] was developed to provide a process to assist manufacturers. Medical Device Risk Management File.
From medicaldevicehq.com
FMEA vs ISO 14971 Medical Device HQ Medical Device Risk Management File introduction to the definitive guide to iso 14971 risk management for medical devices. Conduct risk analysis, where appropriate,. this document specifies terminology, principles and a process for risk management of medical devices,. when to use risk management activities. international standard bs en iso 14971 [1] was developed to provide a process to assist manufacturers in identifying. Medical Device Risk Management File.
From www.researchgate.net
1Medical device risk management strategy (Adapted from ISO 14971 Medical Device Risk Management File international standard bs en iso 14971 [1] was developed to provide a process to assist manufacturers in identifying the hazards. this document specifies terminology, principles and a process for risk management of medical devices,. When implementing a quality system to: introduction to the definitive guide to iso 14971 risk management for medical devices. this document specifies. Medical Device Risk Management File.