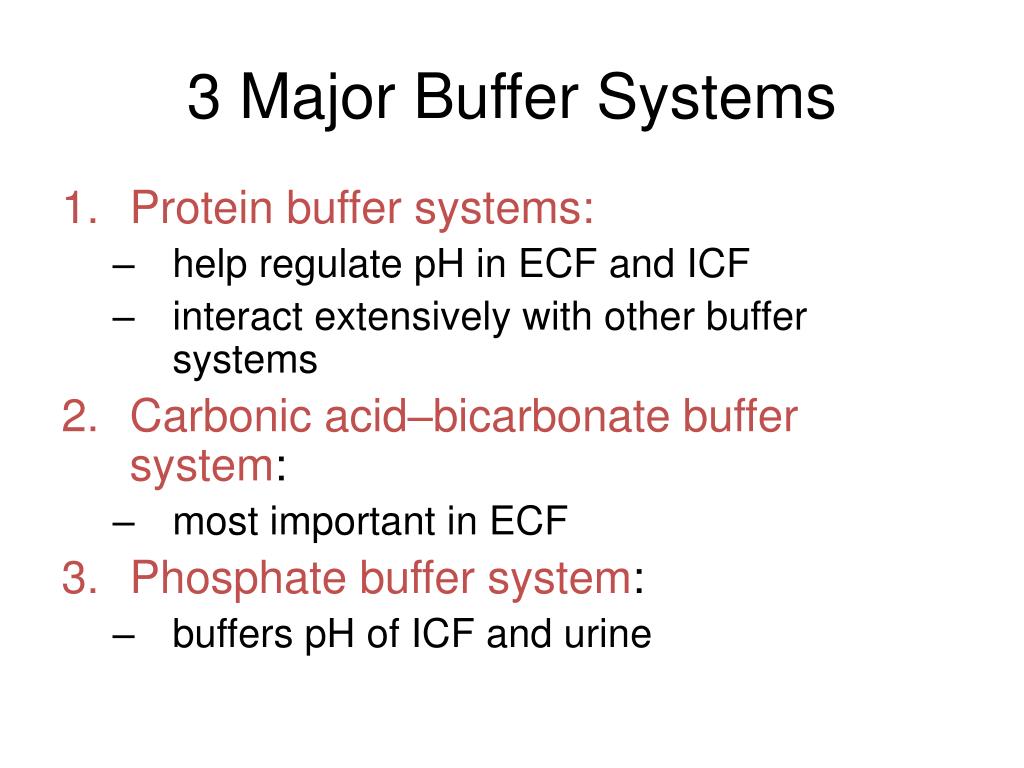
What Is Body Buffer System . It takes only seconds for the chemical buffers in the blood to make. A variety of buffering systems exist in the body that helps maintain the ph of the blood and other fluids within a narrow range—between. A buffer is a solution that resists sudden changes in ph. Buffers are solutions that contain a weak acid and its a conjugate base; All of these contain bases that accept hydrogen. The ph of the blood is maintained between 7.35 and 7.45 by an. A variety of buffering systems permits blood and other bodily fluids to maintain a narrow ph range, even in the face of perturbations. Other buffer systems in the human body include the phosphate buffer system, proteins, and hemoglobin. The buffer systems in the human body are extremely efficient, and different systems work at different rates.
from www.slideserve.com
The ph of the blood is maintained between 7.35 and 7.45 by an. The buffer systems in the human body are extremely efficient, and different systems work at different rates. Other buffer systems in the human body include the phosphate buffer system, proteins, and hemoglobin. A buffer is a solution that resists sudden changes in ph. It takes only seconds for the chemical buffers in the blood to make. A variety of buffering systems exist in the body that helps maintain the ph of the blood and other fluids within a narrow range—between. A variety of buffering systems permits blood and other bodily fluids to maintain a narrow ph range, even in the face of perturbations. All of these contain bases that accept hydrogen. Buffers are solutions that contain a weak acid and its a conjugate base;
PPT Chapter 26 Balance PowerPoint Presentation, free download ID2127603
What Is Body Buffer System It takes only seconds for the chemical buffers in the blood to make. A variety of buffering systems permits blood and other bodily fluids to maintain a narrow ph range, even in the face of perturbations. All of these contain bases that accept hydrogen. A buffer is a solution that resists sudden changes in ph. Buffers are solutions that contain a weak acid and its a conjugate base; The buffer systems in the human body are extremely efficient, and different systems work at different rates. Other buffer systems in the human body include the phosphate buffer system, proteins, and hemoglobin. It takes only seconds for the chemical buffers in the blood to make. The ph of the blood is maintained between 7.35 and 7.45 by an. A variety of buffering systems exist in the body that helps maintain the ph of the blood and other fluids within a narrow range—between.
From www.slideserve.com
PPT Buffer Systems of the Body PowerPoint Presentation, free download ID9427787 What Is Body Buffer System All of these contain bases that accept hydrogen. A variety of buffering systems permits blood and other bodily fluids to maintain a narrow ph range, even in the face of perturbations. Buffers are solutions that contain a weak acid and its a conjugate base; A variety of buffering systems exist in the body that helps maintain the ph of the. What Is Body Buffer System.
From quizlet.com
Bicarbonate Buffer system Diagram Quizlet What Is Body Buffer System Other buffer systems in the human body include the phosphate buffer system, proteins, and hemoglobin. The buffer systems in the human body are extremely efficient, and different systems work at different rates. Buffers are solutions that contain a weak acid and its a conjugate base; All of these contain bases that accept hydrogen. The ph of the blood is maintained. What Is Body Buffer System.
From www.studypool.com
SOLUTION Body buffer system Studypool What Is Body Buffer System A variety of buffering systems permits blood and other bodily fluids to maintain a narrow ph range, even in the face of perturbations. All of these contain bases that accept hydrogen. It takes only seconds for the chemical buffers in the blood to make. The ph of the blood is maintained between 7.35 and 7.45 by an. Buffers are solutions. What Is Body Buffer System.
From www.slideserve.com
PPT Renal Physiology PowerPoint Presentation, free download ID5632772 What Is Body Buffer System Other buffer systems in the human body include the phosphate buffer system, proteins, and hemoglobin. Buffers are solutions that contain a weak acid and its a conjugate base; All of these contain bases that accept hydrogen. It takes only seconds for the chemical buffers in the blood to make. A variety of buffering systems exist in the body that helps. What Is Body Buffer System.
From www.slideserve.com
PPT 1. Body Fluid Compartments PowerPoint Presentation, free download ID6851983 What Is Body Buffer System The ph of the blood is maintained between 7.35 and 7.45 by an. Other buffer systems in the human body include the phosphate buffer system, proteins, and hemoglobin. Buffers are solutions that contain a weak acid and its a conjugate base; A buffer is a solution that resists sudden changes in ph. It takes only seconds for the chemical buffers. What Is Body Buffer System.
From www.pinterest.ca
bicarbonate buffer system, example of multiple equilibria Teaching chemistry, Medical school What Is Body Buffer System The ph of the blood is maintained between 7.35 and 7.45 by an. A variety of buffering systems permits blood and other bodily fluids to maintain a narrow ph range, even in the face of perturbations. Other buffer systems in the human body include the phosphate buffer system, proteins, and hemoglobin. All of these contain bases that accept hydrogen. The. What Is Body Buffer System.
From www.slideshare.net
Buffer system What Is Body Buffer System The buffer systems in the human body are extremely efficient, and different systems work at different rates. A variety of buffering systems exist in the body that helps maintain the ph of the blood and other fluids within a narrow range—between. The ph of the blood is maintained between 7.35 and 7.45 by an. A variety of buffering systems permits. What Is Body Buffer System.
From labpedia.net
Acidbase Balance Part 3 Respiratory Acidosis and Respiratory Alkalosis What Is Body Buffer System It takes only seconds for the chemical buffers in the blood to make. A variety of buffering systems permits blood and other bodily fluids to maintain a narrow ph range, even in the face of perturbations. The ph of the blood is maintained between 7.35 and 7.45 by an. Other buffer systems in the human body include the phosphate buffer. What Is Body Buffer System.
From www.slideserve.com
PPT AcidBase Balance PowerPoint Presentation, free download ID2821926 What Is Body Buffer System The buffer systems in the human body are extremely efficient, and different systems work at different rates. The ph of the blood is maintained between 7.35 and 7.45 by an. Buffers are solutions that contain a weak acid and its a conjugate base; A variety of buffering systems exist in the body that helps maintain the ph of the blood. What Is Body Buffer System.
From www.studypool.com
SOLUTION Pptx body buffer systems aqsa 2 Studypool What Is Body Buffer System A variety of buffering systems exist in the body that helps maintain the ph of the blood and other fluids within a narrow range—between. Other buffer systems in the human body include the phosphate buffer system, proteins, and hemoglobin. A variety of buffering systems permits blood and other bodily fluids to maintain a narrow ph range, even in the face. What Is Body Buffer System.
From www.slideserve.com
PPT Chapter 26 Balance PowerPoint Presentation, free download ID2127603 What Is Body Buffer System The buffer systems in the human body are extremely efficient, and different systems work at different rates. Buffers are solutions that contain a weak acid and its a conjugate base; A buffer is a solution that resists sudden changes in ph. It takes only seconds for the chemical buffers in the blood to make. Other buffer systems in the human. What Is Body Buffer System.
From www.slideserve.com
PPT Chapter 26 Balance PowerPoint Presentation, free download ID2127603 What Is Body Buffer System It takes only seconds for the chemical buffers in the blood to make. The ph of the blood is maintained between 7.35 and 7.45 by an. A variety of buffering systems permits blood and other bodily fluids to maintain a narrow ph range, even in the face of perturbations. Other buffer systems in the human body include the phosphate buffer. What Is Body Buffer System.
From www.slideserve.com
PPT Acids and Bases and Buffers PowerPoint Presentation, free download ID4769309 What Is Body Buffer System Buffers are solutions that contain a weak acid and its a conjugate base; Other buffer systems in the human body include the phosphate buffer system, proteins, and hemoglobin. All of these contain bases that accept hydrogen. It takes only seconds for the chemical buffers in the blood to make. The ph of the blood is maintained between 7.35 and 7.45. What Is Body Buffer System.
From www.slideserve.com
PPT Chapter 26 Balance PowerPoint Presentation, free download ID2127603 What Is Body Buffer System Buffers are solutions that contain a weak acid and its a conjugate base; A buffer is a solution that resists sudden changes in ph. All of these contain bases that accept hydrogen. A variety of buffering systems exist in the body that helps maintain the ph of the blood and other fluids within a narrow range—between. The ph of the. What Is Body Buffer System.
From www.slideserve.com
PPT Buffers of Biological & Clinical Significance PowerPoint Presentation ID5857540 What Is Body Buffer System The buffer systems in the human body are extremely efficient, and different systems work at different rates. It takes only seconds for the chemical buffers in the blood to make. A buffer is a solution that resists sudden changes in ph. Other buffer systems in the human body include the phosphate buffer system, proteins, and hemoglobin. All of these contain. What Is Body Buffer System.
From www.studocu.com
Biological Buffer Systems BIOLOGICAL BUFFER SYSTEMS Almost every biological process is Studocu What Is Body Buffer System The buffer systems in the human body are extremely efficient, and different systems work at different rates. Buffers are solutions that contain a weak acid and its a conjugate base; It takes only seconds for the chemical buffers in the blood to make. The ph of the blood is maintained between 7.35 and 7.45 by an. A variety of buffering. What Is Body Buffer System.
From bazaarstory.blogspot.com
Plasma Protein Buffer System bazaarstory What Is Body Buffer System The ph of the blood is maintained between 7.35 and 7.45 by an. Buffers are solutions that contain a weak acid and its a conjugate base; The buffer systems in the human body are extremely efficient, and different systems work at different rates. A variety of buffering systems exist in the body that helps maintain the ph of the blood. What Is Body Buffer System.
From www.slideserve.com
PPT Buffer Systems of the Body PowerPoint Presentation, free download ID4264848 What Is Body Buffer System The buffer systems in the human body are extremely efficient, and different systems work at different rates. Other buffer systems in the human body include the phosphate buffer system, proteins, and hemoglobin. It takes only seconds for the chemical buffers in the blood to make. All of these contain bases that accept hydrogen. A buffer is a solution that resists. What Is Body Buffer System.
From studylib.net
Body Buffer Systems What Is Body Buffer System The ph of the blood is maintained between 7.35 and 7.45 by an. All of these contain bases that accept hydrogen. A variety of buffering systems permits blood and other bodily fluids to maintain a narrow ph range, even in the face of perturbations. It takes only seconds for the chemical buffers in the blood to make. The buffer systems. What Is Body Buffer System.
From www.slideshare.net
Buffer system What Is Body Buffer System It takes only seconds for the chemical buffers in the blood to make. Other buffer systems in the human body include the phosphate buffer system, proteins, and hemoglobin. All of these contain bases that accept hydrogen. A variety of buffering systems exist in the body that helps maintain the ph of the blood and other fluids within a narrow range—between.. What Is Body Buffer System.
From www.slideserve.com
PPT Homeostasis PowerPoint Presentation, free download ID1138529 What Is Body Buffer System A variety of buffering systems permits blood and other bodily fluids to maintain a narrow ph range, even in the face of perturbations. The ph of the blood is maintained between 7.35 and 7.45 by an. Buffers are solutions that contain a weak acid and its a conjugate base; All of these contain bases that accept hydrogen. A variety of. What Is Body Buffer System.
From www.slideserve.com
PPT Water & Buffers PowerPoint Presentation, free download ID3886735 What Is Body Buffer System Other buffer systems in the human body include the phosphate buffer system, proteins, and hemoglobin. A variety of buffering systems permits blood and other bodily fluids to maintain a narrow ph range, even in the face of perturbations. The buffer systems in the human body are extremely efficient, and different systems work at different rates. The ph of the blood. What Is Body Buffer System.
From www.slideshare.net
Buffer system What Is Body Buffer System A variety of buffering systems exist in the body that helps maintain the ph of the blood and other fluids within a narrow range—between. The ph of the blood is maintained between 7.35 and 7.45 by an. A variety of buffering systems permits blood and other bodily fluids to maintain a narrow ph range, even in the face of perturbations.. What Is Body Buffer System.
From www.youtube.com
Introduction to Buffer System Regulation of pH Acid Base Balance Buffers in What Is Body Buffer System All of these contain bases that accept hydrogen. The ph of the blood is maintained between 7.35 and 7.45 by an. A variety of buffering systems permits blood and other bodily fluids to maintain a narrow ph range, even in the face of perturbations. Buffers are solutions that contain a weak acid and its a conjugate base; Other buffer systems. What Is Body Buffer System.
From www.slideserve.com
PPT Chapter 27 Fluid, Electrolyte and Acidbase Balance PowerPoint Presentation ID2687569 What Is Body Buffer System A variety of buffering systems exist in the body that helps maintain the ph of the blood and other fluids within a narrow range—between. The ph of the blood is maintained between 7.35 and 7.45 by an. All of these contain bases that accept hydrogen. A variety of buffering systems permits blood and other bodily fluids to maintain a narrow. What Is Body Buffer System.
From www.slideshare.net
Acid base balance What Is Body Buffer System Buffers are solutions that contain a weak acid and its a conjugate base; A variety of buffering systems permits blood and other bodily fluids to maintain a narrow ph range, even in the face of perturbations. A variety of buffering systems exist in the body that helps maintain the ph of the blood and other fluids within a narrow range—between.. What Is Body Buffer System.
From ridtpoof-lano.blogspot.com
What Is The Most Powerful Buffer System In The Body Maria Ma Coiffure What Is Body Buffer System A variety of buffering systems exist in the body that helps maintain the ph of the blood and other fluids within a narrow range—between. Buffers are solutions that contain a weak acid and its a conjugate base; Other buffer systems in the human body include the phosphate buffer system, proteins, and hemoglobin. The ph of the blood is maintained between. What Is Body Buffer System.
From ridtpoof-lano.blogspot.com
What Is The Most Powerful Buffer System In The Body Maria Ma Coiffure What Is Body Buffer System Other buffer systems in the human body include the phosphate buffer system, proteins, and hemoglobin. A buffer is a solution that resists sudden changes in ph. Buffers are solutions that contain a weak acid and its a conjugate base; The buffer systems in the human body are extremely efficient, and different systems work at different rates. All of these contain. What Is Body Buffer System.
From www.slideserve.com
PPT Unit Five The Body Fluids and Kidneys PowerPoint Presentation, free download ID3308641 What Is Body Buffer System A variety of buffering systems exist in the body that helps maintain the ph of the blood and other fluids within a narrow range—between. A variety of buffering systems permits blood and other bodily fluids to maintain a narrow ph range, even in the face of perturbations. All of these contain bases that accept hydrogen. It takes only seconds for. What Is Body Buffer System.
From www.slideserve.com
PPT Physiological system of blood. Functional importance of blood plasma components What Is Body Buffer System It takes only seconds for the chemical buffers in the blood to make. The ph of the blood is maintained between 7.35 and 7.45 by an. The buffer systems in the human body are extremely efficient, and different systems work at different rates. All of these contain bases that accept hydrogen. A buffer is a solution that resists sudden changes. What Is Body Buffer System.
From www.pinterest.com
Physiology Blood Buffer System Behrouz Human body facts, Biochemistry, Physiology What Is Body Buffer System Other buffer systems in the human body include the phosphate buffer system, proteins, and hemoglobin. The buffer systems in the human body are extremely efficient, and different systems work at different rates. The ph of the blood is maintained between 7.35 and 7.45 by an. A buffer is a solution that resists sudden changes in ph. It takes only seconds. What Is Body Buffer System.
From www.slideserve.com
PPT Blood Gases, pH and Buffer system PowerPoint Presentation ID257615 What Is Body Buffer System The buffer systems in the human body are extremely efficient, and different systems work at different rates. The ph of the blood is maintained between 7.35 and 7.45 by an. It takes only seconds for the chemical buffers in the blood to make. A variety of buffering systems exist in the body that helps maintain the ph of the blood. What Is Body Buffer System.
From www.slideshare.net
Buffer system What Is Body Buffer System The buffer systems in the human body are extremely efficient, and different systems work at different rates. All of these contain bases that accept hydrogen. Other buffer systems in the human body include the phosphate buffer system, proteins, and hemoglobin. A variety of buffering systems permits blood and other bodily fluids to maintain a narrow ph range, even in the. What Is Body Buffer System.
From poorvi77.blogspot.com
Poorveez ParadyzMedimuseion major buffer systems in the body What Is Body Buffer System It takes only seconds for the chemical buffers in the blood to make. Buffers are solutions that contain a weak acid and its a conjugate base; All of these contain bases that accept hydrogen. A buffer is a solution that resists sudden changes in ph. Other buffer systems in the human body include the phosphate buffer system, proteins, and hemoglobin.. What Is Body Buffer System.
From www.slideserve.com
PPT AcidBase Homeostasis PowerPoint Presentation, free download ID3309212 What Is Body Buffer System Buffers are solutions that contain a weak acid and its a conjugate base; A buffer is a solution that resists sudden changes in ph. A variety of buffering systems permits blood and other bodily fluids to maintain a narrow ph range, even in the face of perturbations. The buffer systems in the human body are extremely efficient, and different systems. What Is Body Buffer System.