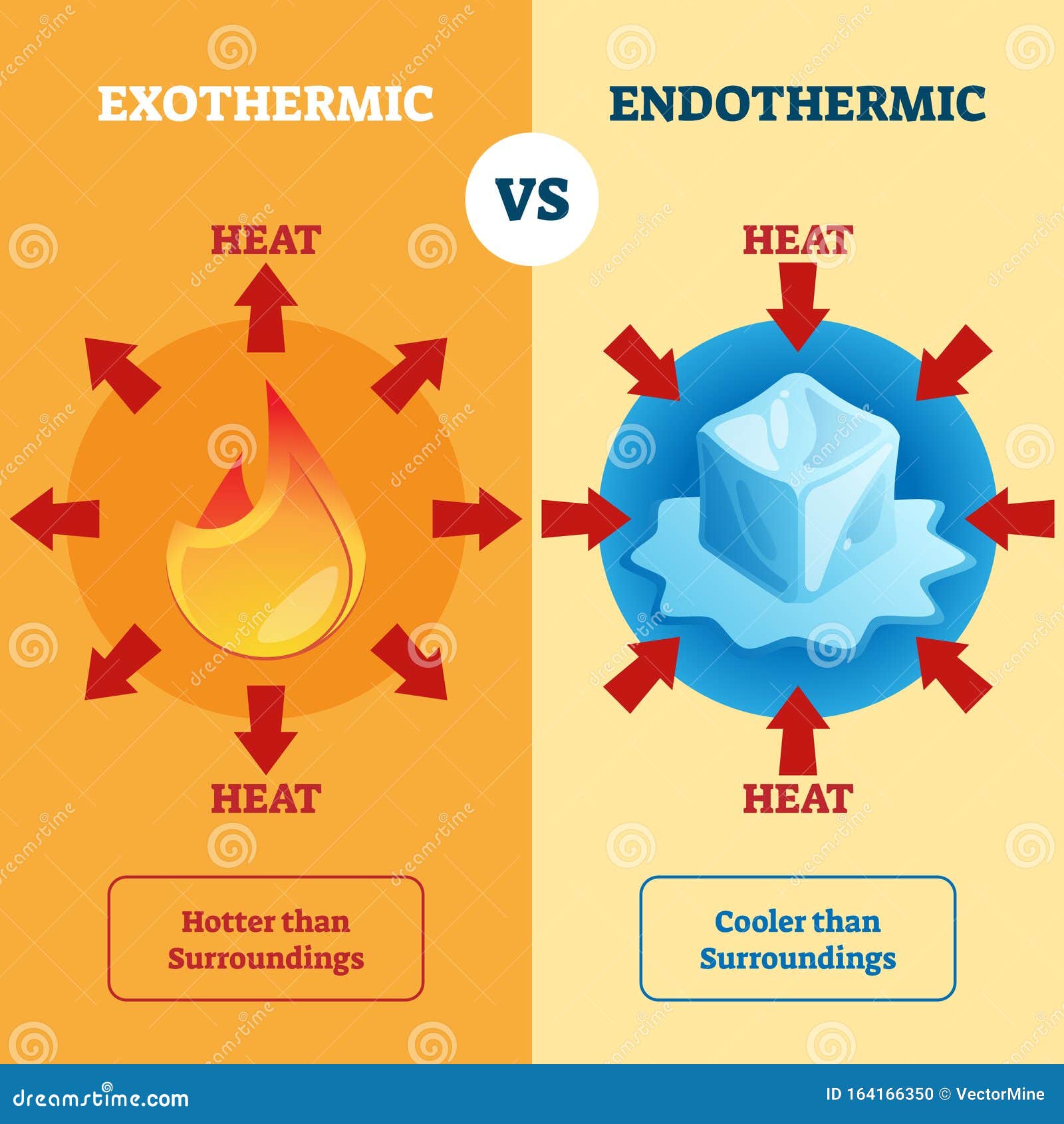
Endothermic Reaction To Water . Determine whether a reaction is endothermic or exothermic through observations,. An endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. Determine if a chemical process is exothermic or endothermic. one of your salts generated an endothermic reaction with water, while the other salt generated an exothermic reaction with water. examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold packs. in an endothermic process, the heat that a system absorbs is thermal energy transfer into the system. the reactants transform into products. dissolving ammonium nitrate in water is endothermic because more energy is used to pull the ions in the solid apart than is produced when the ions form new bonds with water molecules. Let me first reveal the identity of your salts: Describe how heat is transferred in endothermic and exothermic reactions. define endothermic and exothermic reactions. Use bond dissociation energies to calculate enthalpy change or heat of reaction. When ammonium chloride (nh 4 cl) is dissolved in water, an endothermic reaction takes place. They absorb heat energy to overcome the activation energy barrier that allows the reaction to form products. Most endothermic reactions are not spontaneous, i.e., the.
from www.myxxgirl.com
in an endothermic process, the heat that a system absorbs is thermal energy transfer into the system. Describe how heat is transferred in endothermic and exothermic reactions. the reactants transform into products. Use bond dissociation energies to calculate enthalpy change or heat of reaction. examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold packs. Determine if a chemical process is exothermic or endothermic. define endothermic and exothermic reactions. They absorb heat energy to overcome the activation energy barrier that allows the reaction to form products. dissolving ammonium nitrate in water is endothermic because more energy is used to pull the ions in the solid apart than is produced when the ions form new bonds with water molecules. When ammonium chloride (nh 4 cl) is dissolved in water, an endothermic reaction takes place.
Endothermic Vs Exothermic Reactions Infographic Diagram Cartoon Vector
Endothermic Reaction To Water When ammonium chloride (nh 4 cl) is dissolved in water, an endothermic reaction takes place. one of your salts generated an endothermic reaction with water, while the other salt generated an exothermic reaction with water. Let me first reveal the identity of your salts: Thus, an endothermic reaction generally leads to an increase in the temperature of the system and a decrease. the reactants transform into products. Most endothermic reactions are not spontaneous, i.e., the. An endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. define endothermic and exothermic reactions. examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold packs. Determine if a chemical process is exothermic or endothermic. Describe how heat is transferred in endothermic and exothermic reactions. dissolving ammonium nitrate in water is endothermic because more energy is used to pull the ions in the solid apart than is produced when the ions form new bonds with water molecules. Salt a is ammonium nitrate ( nh 4 no 3 ). Use bond dissociation energies to calculate enthalpy change or heat of reaction. Determine whether a reaction is endothermic or exothermic through observations,. When ammonium chloride (nh 4 cl) is dissolved in water, an endothermic reaction takes place.
From inberp.info
Differences between exothermic and endothermic reactions (2023) Endothermic Reaction To Water Most endothermic reactions are not spontaneous, i.e., the. examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold packs. in an endothermic process, the heat that a system absorbs is thermal energy transfer into the system. Determine if a chemical process is exothermic or endothermic. define endothermic and exothermic reactions. An endothermic reaction is. Endothermic Reaction To Water.
From stock.adobe.com
Activation energy in endothermic and exothermic reactions. Stock Endothermic Reaction To Water define endothermic and exothermic reactions. Determine if a chemical process is exothermic or endothermic. the reactants transform into products. dissolving ammonium nitrate in water is endothermic because more energy is used to pull the ions in the solid apart than is produced when the ions form new bonds with water molecules. They absorb heat energy to overcome. Endothermic Reaction To Water.
From www.aiophotoz.com
Endothermic Reaction Diagram Labeled Diagram Media Images and Photos Endothermic Reaction To Water Let me first reveal the identity of your salts: examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold packs. in an endothermic process, the heat that a system absorbs is thermal energy transfer into the system. dissolving ammonium nitrate in water is endothermic because more energy is used to pull the ions in. Endothermic Reaction To Water.
From www.animalia-life.club
Endothermic And Exothermic Reaction Examples Endothermic Reaction To Water Salt a is ammonium nitrate ( nh 4 no 3 ). the reactants transform into products. Thus, an endothermic reaction generally leads to an increase in the temperature of the system and a decrease. When ammonium chloride (nh 4 cl) is dissolved in water, an endothermic reaction takes place. Determine if a chemical process is exothermic or endothermic.. Endothermic Reaction To Water.
From vhmsscience.weebly.com
Endo/Exothermic Reactions VISTA HEIGHTS 8TH GRADE SCIENCE Endothermic Reaction To Water An endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold packs. one of your salts generated an endothermic reaction with water, while the other salt generated an exothermic reaction with water. define endothermic and exothermic reactions. Most endothermic reactions are. Endothermic Reaction To Water.
From www.teachoo.com
Which of the reactions is an endothermic reaction? MCQ Science Endothermic Reaction To Water Salt a is ammonium nitrate ( nh 4 no 3 ). Thus, an endothermic reaction generally leads to an increase in the temperature of the system and a decrease. Describe how heat is transferred in endothermic and exothermic reactions. Let me first reveal the identity of your salts: the reactants transform into products. define endothermic and exothermic. Endothermic Reaction To Water.
From trendqcloud.blogspot.com
View 9 Endothermic Reaction Diagram trendqcloud Endothermic Reaction To Water Most endothermic reactions are not spontaneous, i.e., the. examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold packs. Determine if a chemical process is exothermic or endothermic. in an endothermic process, the heat that a system absorbs is thermal energy transfer into the system. dissolving ammonium nitrate in water is endothermic because more. Endothermic Reaction To Water.
From www.chemistrylearner.com
Endothermic Reaction Definition, Equation, Graph & Examples Endothermic Reaction To Water When ammonium chloride (nh 4 cl) is dissolved in water, an endothermic reaction takes place. in an endothermic process, the heat that a system absorbs is thermal energy transfer into the system. They absorb heat energy to overcome the activation energy barrier that allows the reaction to form products. Most endothermic reactions are not spontaneous, i.e., the. dissolving. Endothermic Reaction To Water.
From sciencestruck.com
Endothermic Reaction Examples Science Struck Endothermic Reaction To Water Thus, an endothermic reaction generally leads to an increase in the temperature of the system and a decrease. Determine whether a reaction is endothermic or exothermic through observations,. define endothermic and exothermic reactions. An endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. dissolving ammonium nitrate in water is endothermic because more energy is. Endothermic Reaction To Water.
From www.myxxgirl.com
Endothermic Vs Exothermic Reactions Infographic Diagram Cartoon Vector Endothermic Reaction To Water Salt a is ammonium nitrate ( nh 4 no 3 ). Determine if a chemical process is exothermic or endothermic. Thus, an endothermic reaction generally leads to an increase in the temperature of the system and a decrease. An endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. Let me first reveal the identity of. Endothermic Reaction To Water.
From revisechemistry.uk
Exothermic and Endothermic Reactions AQA C5 revisechemistry.uk Endothermic Reaction To Water in an endothermic process, the heat that a system absorbs is thermal energy transfer into the system. Most endothermic reactions are not spontaneous, i.e., the. Determine if a chemical process is exothermic or endothermic. Salt a is ammonium nitrate ( nh 4 no 3 ). examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical. Endothermic Reaction To Water.
From vhmsscience.weebly.com
Endo/Exothermic Reactions VISTA HEIGHTS 8TH GRADE SCIENCE Endothermic Reaction To Water Determine if a chemical process is exothermic or endothermic. When ammonium chloride (nh 4 cl) is dissolved in water, an endothermic reaction takes place. An endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold packs. the reactants transform into products. . Endothermic Reaction To Water.
From chhattisgarh.pscnotes.com
Exothermic and endothermic reactions CGPCS Exam Preparation Endothermic Reaction To Water in an endothermic process, the heat that a system absorbs is thermal energy transfer into the system. When ammonium chloride (nh 4 cl) is dissolved in water, an endothermic reaction takes place. Use bond dissociation energies to calculate enthalpy change or heat of reaction. examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold packs.. Endothermic Reaction To Water.
From vhmsscience.weebly.com
Endo/Exothermic Reactions VISTA HEIGHTS 8TH GRADE SCIENCE Endothermic Reaction To Water the reactants transform into products. Describe how heat is transferred in endothermic and exothermic reactions. Thus, an endothermic reaction generally leads to an increase in the temperature of the system and a decrease. They absorb heat energy to overcome the activation energy barrier that allows the reaction to form products. examples of endothermic reactions include photosynthesis, dissolving salt. Endothermic Reaction To Water.
From classnotes123.com
What does one mean by exothermic and endothermic reactions? Give Endothermic Reaction To Water Let me first reveal the identity of your salts: Salt a is ammonium nitrate ( nh 4 no 3 ). define endothermic and exothermic reactions. Determine whether a reaction is endothermic or exothermic through observations,. examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold packs. Determine if a chemical process is exothermic or. Endothermic Reaction To Water.
From www.diffzy.com
Exothermic vs. Endothermic Reactions What's the Difference (With Table) Endothermic Reaction To Water Use bond dissociation energies to calculate enthalpy change or heat of reaction. Salt a is ammonium nitrate ( nh 4 no 3 ). define endothermic and exothermic reactions. Describe how heat is transferred in endothermic and exothermic reactions. in an endothermic process, the heat that a system absorbs is thermal energy transfer into the system. They absorb. Endothermic Reaction To Water.
From www.chemistrylearner.com
Endothermic Reaction Definition, Equation, Graph & Examples Endothermic Reaction To Water An endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. the reactants transform into products. Use bond dissociation energies to calculate enthalpy change or heat of reaction. in an endothermic process, the heat that a system absorbs is thermal energy transfer into the system. Thus, an endothermic reaction generally leads to an increase in. Endothermic Reaction To Water.
From app.pandai.org
Thermochemistry Endothermic Reaction To Water dissolving ammonium nitrate in water is endothermic because more energy is used to pull the ions in the solid apart than is produced when the ions form new bonds with water molecules. When ammonium chloride (nh 4 cl) is dissolved in water, an endothermic reaction takes place. Describe how heat is transferred in endothermic and exothermic reactions. Determine whether. Endothermic Reaction To Water.
From www.animalia-life.club
Endothermic And Exothermic Reaction Examples Endothermic Reaction To Water An endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. They absorb heat energy to overcome the activation energy barrier that allows the reaction to form products. Thus, an endothermic reaction generally leads to an increase in the temperature of the system and a decrease. examples of endothermic reactions include photosynthesis, dissolving salt in water,. Endothermic Reaction To Water.
From www.coursehero.com
What is the equilibrium constant for a reaction that has a value of Endothermic Reaction To Water When ammonium chloride (nh 4 cl) is dissolved in water, an endothermic reaction takes place. one of your salts generated an endothermic reaction with water, while the other salt generated an exothermic reaction with water. Use bond dissociation energies to calculate enthalpy change or heat of reaction. An endothermic reaction is a chemical reaction that absorbs thermal energy from. Endothermic Reaction To Water.
From www.studyorgo.com
How to Interpret Thermodynamics of Reactions Endothermic Reaction To Water the reactants transform into products. Most endothermic reactions are not spontaneous, i.e., the. Use bond dissociation energies to calculate enthalpy change or heat of reaction. An endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. Determine whether a reaction is endothermic or exothermic through observations,. Let me first reveal the identity of your salts: Thus,. Endothermic Reaction To Water.
From sciencenotes.org
Endothermic Reactions Definition and Examples Endothermic Reaction To Water in an endothermic process, the heat that a system absorbs is thermal energy transfer into the system. examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold packs. one of your salts generated an endothermic reaction with water, while the other salt generated an exothermic reaction with water. Most endothermic reactions are not spontaneous,. Endothermic Reaction To Water.
From online-learning-college.com
Exothermic and endothermic reactions Key differences Endothermic Reaction To Water Let me first reveal the identity of your salts: Salt a is ammonium nitrate ( nh 4 no 3 ). When ammonium chloride (nh 4 cl) is dissolved in water, an endothermic reaction takes place. in an endothermic process, the heat that a system absorbs is thermal energy transfer into the system. Determine whether a reaction is endothermic. Endothermic Reaction To Water.
From www.thoughtco.com
Endothermic and Exothermic Chemical Reactions Endothermic Reaction To Water define endothermic and exothermic reactions. An endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. Thus, an endothermic reaction generally leads to an increase in the temperature of the system and a decrease. Determine whether a reaction is endothermic or exothermic through observations,. examples of endothermic reactions include photosynthesis, dissolving salt in water, and. Endothermic Reaction To Water.
From www.slideserve.com
PPT Exothermic and endothermic reactions PowerPoint Presentation Endothermic Reaction To Water define endothermic and exothermic reactions. Describe how heat is transferred in endothermic and exothermic reactions. Let me first reveal the identity of your salts: An endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. Thus, an endothermic reaction generally leads to an increase in the temperature of the system and a decrease. Determine whether a. Endothermic Reaction To Water.
From www.slideserve.com
PPT Endothermic Vs. Exothermic Reaction Graphs PowerPoint Endothermic Reaction To Water An endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. Use bond dissociation energies to calculate enthalpy change or heat of reaction. dissolving ammonium nitrate in water is endothermic because more energy is used to pull the ions in the solid apart than is produced when the ions form new bonds with water molecules. Describe. Endothermic Reaction To Water.
From sciencenotes.org
Endothermic Reactions Definition and Examples Endothermic Reaction To Water in an endothermic process, the heat that a system absorbs is thermal energy transfer into the system. Most endothermic reactions are not spontaneous, i.e., the. the reactants transform into products. Describe how heat is transferred in endothermic and exothermic reactions. Determine whether a reaction is endothermic or exothermic through observations,. define endothermic and exothermic reactions. Determine if. Endothermic Reaction To Water.
From examples.yourdictionary.com
Exothermic Reaction Examples Found in Real Life Endothermic Reaction To Water Let me first reveal the identity of your salts: in an endothermic process, the heat that a system absorbs is thermal energy transfer into the system. define endothermic and exothermic reactions. dissolving ammonium nitrate in water is endothermic because more energy is used to pull the ions in the solid apart than is produced when the ions. Endothermic Reaction To Water.
From byjus.com
Difference Between Endothermic and Exothermic Reactions Chemistry Endothermic Reaction To Water They absorb heat energy to overcome the activation energy barrier that allows the reaction to form products. examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold packs. Determine whether a reaction is endothermic or exothermic through observations,. dissolving ammonium nitrate in water is endothermic because more energy is used to pull the ions in. Endothermic Reaction To Water.
From pixels.com
Exothermic And Endothermic Chemical Reactions Photograph by Inna Bigun Endothermic Reaction To Water Let me first reveal the identity of your salts: examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold packs. the reactants transform into products. An endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. one of your salts generated an endothermic reaction with water, while the other salt generated. Endothermic Reaction To Water.
From courses.lumenlearning.com
Exothermic and Endothermic Processes Introduction to Chemistry Endothermic Reaction To Water Salt a is ammonium nitrate ( nh 4 no 3 ). examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold packs. Most endothermic reactions are not spontaneous, i.e., the. They absorb heat energy to overcome the activation energy barrier that allows the reaction to form products. Use bond dissociation energies to calculate enthalpy change. Endothermic Reaction To Water.
From www.aiophotoz.com
What Is The Difference Between Exothermic And Endothermic Chemical Endothermic Reaction To Water Most endothermic reactions are not spontaneous, i.e., the. They absorb heat energy to overcome the activation energy barrier that allows the reaction to form products. define endothermic and exothermic reactions. Determine whether a reaction is endothermic or exothermic through observations,. Thus, an endothermic reaction generally leads to an increase in the temperature of the system and a decrease. Determine. Endothermic Reaction To Water.
From chemistai.org
What is computational chemistry? Endothermic Reaction To Water Thus, an endothermic reaction generally leads to an increase in the temperature of the system and a decrease. Determine if a chemical process is exothermic or endothermic. Use bond dissociation energies to calculate enthalpy change or heat of reaction. Describe how heat is transferred in endothermic and exothermic reactions. in an endothermic process, the heat that a system absorbs. Endothermic Reaction To Water.
From www.animalia-life.club
Endothermic Reaction Examples For Kids Endothermic Reaction To Water Describe how heat is transferred in endothermic and exothermic reactions. Determine whether a reaction is endothermic or exothermic through observations,. in an endothermic process, the heat that a system absorbs is thermal energy transfer into the system. Use bond dissociation energies to calculate enthalpy change or heat of reaction. When ammonium chloride (nh 4 cl) is dissolved in water,. Endothermic Reaction To Water.
From www.thoughtco.com
Endothermic Reaction Examples Endothermic Reaction To Water in an endothermic process, the heat that a system absorbs is thermal energy transfer into the system. Use bond dissociation energies to calculate enthalpy change or heat of reaction. Salt a is ammonium nitrate ( nh 4 no 3 ). Most endothermic reactions are not spontaneous, i.e., the. dissolving ammonium nitrate in water is endothermic because more. Endothermic Reaction To Water.