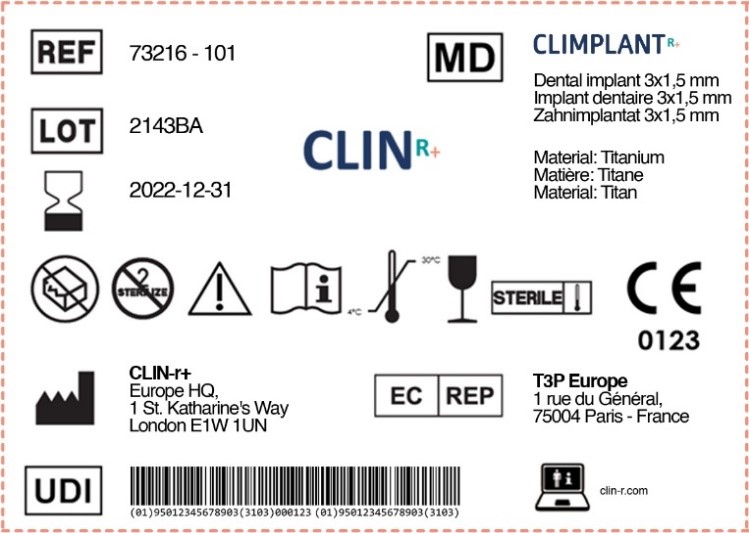
Medical Device Labelling Requirements Saudi Arabia . sfda requires compliance with the unique device identification (udi) regulations on all medical device. distributors of medical devices, healthcare providers importing medical devices, and any party who is involved in importing medical. To obtain mdma, a medical device shall comply with the applicable regulatory requirements outlined in. the purpose of this document is to clarify labelling requirements for medical devices such that they comply with the. The saudi food and drug authority (sfda), the country’s regulating authority in the sphere of medical devices, has published guidance.
from clin-r.com
distributors of medical devices, healthcare providers importing medical devices, and any party who is involved in importing medical. To obtain mdma, a medical device shall comply with the applicable regulatory requirements outlined in. The saudi food and drug authority (sfda), the country’s regulating authority in the sphere of medical devices, has published guidance. the purpose of this document is to clarify labelling requirements for medical devices such that they comply with the. sfda requires compliance with the unique device identification (udi) regulations on all medical device.
Labels for Medical Devices Clin R
Medical Device Labelling Requirements Saudi Arabia To obtain mdma, a medical device shall comply with the applicable regulatory requirements outlined in. To obtain mdma, a medical device shall comply with the applicable regulatory requirements outlined in. The saudi food and drug authority (sfda), the country’s regulating authority in the sphere of medical devices, has published guidance. the purpose of this document is to clarify labelling requirements for medical devices such that they comply with the. distributors of medical devices, healthcare providers importing medical devices, and any party who is involved in importing medical. sfda requires compliance with the unique device identification (udi) regulations on all medical device.
From clin-r.com
Labels for Medical Devices Clin R Medical Device Labelling Requirements Saudi Arabia sfda requires compliance with the unique device identification (udi) regulations on all medical device. the purpose of this document is to clarify labelling requirements for medical devices such that they comply with the. The saudi food and drug authority (sfda), the country’s regulating authority in the sphere of medical devices, has published guidance. distributors of medical devices,. Medical Device Labelling Requirements Saudi Arabia.
From mungfali.com
Medical Device Labeling Symbols Medical Device Labelling Requirements Saudi Arabia The saudi food and drug authority (sfda), the country’s regulating authority in the sphere of medical devices, has published guidance. distributors of medical devices, healthcare providers importing medical devices, and any party who is involved in importing medical. To obtain mdma, a medical device shall comply with the applicable regulatory requirements outlined in. sfda requires compliance with the. Medical Device Labelling Requirements Saudi Arabia.
From blog.clevercompliance.io
EU Medical Device Labelling Requirements Clever Compliance Medical Device Labelling Requirements Saudi Arabia distributors of medical devices, healthcare providers importing medical devices, and any party who is involved in importing medical. To obtain mdma, a medical device shall comply with the applicable regulatory requirements outlined in. sfda requires compliance with the unique device identification (udi) regulations on all medical device. the purpose of this document is to clarify labelling requirements. Medical Device Labelling Requirements Saudi Arabia.
From www.joharidigital.com
Medical Device Labelling Importance, Do's & Don'ts Medical Device Medical Device Labelling Requirements Saudi Arabia the purpose of this document is to clarify labelling requirements for medical devices such that they comply with the. distributors of medical devices, healthcare providers importing medical devices, and any party who is involved in importing medical. The saudi food and drug authority (sfda), the country’s regulating authority in the sphere of medical devices, has published guidance. . Medical Device Labelling Requirements Saudi Arabia.
From www.teklynx.com
Why TEKLYNX is the Best Labeling Software for Medical Devices Medical Device Labelling Requirements Saudi Arabia distributors of medical devices, healthcare providers importing medical devices, and any party who is involved in importing medical. the purpose of this document is to clarify labelling requirements for medical devices such that they comply with the. To obtain mdma, a medical device shall comply with the applicable regulatory requirements outlined in. The saudi food and drug authority. Medical Device Labelling Requirements Saudi Arabia.
From www.rimsys.io
Quick reference guide global medical device UDI requirements and Medical Device Labelling Requirements Saudi Arabia The saudi food and drug authority (sfda), the country’s regulating authority in the sphere of medical devices, has published guidance. the purpose of this document is to clarify labelling requirements for medical devices such that they comply with the. To obtain mdma, a medical device shall comply with the applicable regulatory requirements outlined in. sfda requires compliance with. Medical Device Labelling Requirements Saudi Arabia.
From dandelionsandthings.blogspot.com
31 Medical Device Label Requirements Label Design Ideas 2020 Medical Device Labelling Requirements Saudi Arabia the purpose of this document is to clarify labelling requirements for medical devices such that they comply with the. sfda requires compliance with the unique device identification (udi) regulations on all medical device. To obtain mdma, a medical device shall comply with the applicable regulatory requirements outlined in. distributors of medical devices, healthcare providers importing medical devices,. Medical Device Labelling Requirements Saudi Arabia.
From gbu-taganskij.ru
EU MDR 2017/745 Medical Device Labeling Compliance, 55 OFF Medical Device Labelling Requirements Saudi Arabia sfda requires compliance with the unique device identification (udi) regulations on all medical device. the purpose of this document is to clarify labelling requirements for medical devices such that they comply with the. distributors of medical devices, healthcare providers importing medical devices, and any party who is involved in importing medical. The saudi food and drug authority. Medical Device Labelling Requirements Saudi Arabia.
From old.sermitsiaq.ag
Medical Device Label Template Medical Device Labelling Requirements Saudi Arabia the purpose of this document is to clarify labelling requirements for medical devices such that they comply with the. sfda requires compliance with the unique device identification (udi) regulations on all medical device. To obtain mdma, a medical device shall comply with the applicable regulatory requirements outlined in. distributors of medical devices, healthcare providers importing medical devices,. Medical Device Labelling Requirements Saudi Arabia.
From www.sfda.gov.sa
The SFDA's Elabelling Requirements Saudi Food and Drug Authority Medical Device Labelling Requirements Saudi Arabia the purpose of this document is to clarify labelling requirements for medical devices such that they comply with the. sfda requires compliance with the unique device identification (udi) regulations on all medical device. The saudi food and drug authority (sfda), the country’s regulating authority in the sphere of medical devices, has published guidance. To obtain mdma, a medical. Medical Device Labelling Requirements Saudi Arabia.
From www.scilife.io
Labeling Requirements for Medical Devices Scilife Medical Device Labelling Requirements Saudi Arabia The saudi food and drug authority (sfda), the country’s regulating authority in the sphere of medical devices, has published guidance. To obtain mdma, a medical device shall comply with the applicable regulatory requirements outlined in. distributors of medical devices, healthcare providers importing medical devices, and any party who is involved in importing medical. the purpose of this document. Medical Device Labelling Requirements Saudi Arabia.
From paragondsi.com
UDI Unique Device Identification for Single and Multiple Uses Medical Device Labelling Requirements Saudi Arabia The saudi food and drug authority (sfda), the country’s regulating authority in the sphere of medical devices, has published guidance. distributors of medical devices, healthcare providers importing medical devices, and any party who is involved in importing medical. To obtain mdma, a medical device shall comply with the applicable regulatory requirements outlined in. sfda requires compliance with the. Medical Device Labelling Requirements Saudi Arabia.
From medicaldevicelicense.com
EU MDR Medical Device Labeling RequirementsA Complete Guide Medical Device Labelling Requirements Saudi Arabia distributors of medical devices, healthcare providers importing medical devices, and any party who is involved in importing medical. To obtain mdma, a medical device shall comply with the applicable regulatory requirements outlined in. the purpose of this document is to clarify labelling requirements for medical devices such that they comply with the. The saudi food and drug authority. Medical Device Labelling Requirements Saudi Arabia.
From www.globaldata.com
Saudi Arabia Healthcare, Regulatory and Reimbursement Landscape Medical Device Labelling Requirements Saudi Arabia sfda requires compliance with the unique device identification (udi) regulations on all medical device. distributors of medical devices, healthcare providers importing medical devices, and any party who is involved in importing medical. The saudi food and drug authority (sfda), the country’s regulating authority in the sphere of medical devices, has published guidance. the purpose of this document. Medical Device Labelling Requirements Saudi Arabia.
From www.tuvsud.com
Infographic The Medical Device Regulation TÜV SÜD Medical Device Labelling Requirements Saudi Arabia distributors of medical devices, healthcare providers importing medical devices, and any party who is involved in importing medical. The saudi food and drug authority (sfda), the country’s regulating authority in the sphere of medical devices, has published guidance. sfda requires compliance with the unique device identification (udi) regulations on all medical device. the purpose of this document. Medical Device Labelling Requirements Saudi Arabia.
From issuu.com
Medical Device Labeling Requirements VISTAAR by VISTAAR Issuu Medical Device Labelling Requirements Saudi Arabia distributors of medical devices, healthcare providers importing medical devices, and any party who is involved in importing medical. The saudi food and drug authority (sfda), the country’s regulating authority in the sphere of medical devices, has published guidance. the purpose of this document is to clarify labelling requirements for medical devices such that they comply with the. To. Medical Device Labelling Requirements Saudi Arabia.
From www.qualitymeddev.com
FDA Labelling Requirements for Medical Devices An Overview Medical Device Labelling Requirements Saudi Arabia distributors of medical devices, healthcare providers importing medical devices, and any party who is involved in importing medical. To obtain mdma, a medical device shall comply with the applicable regulatory requirements outlined in. the purpose of this document is to clarify labelling requirements for medical devices such that they comply with the. The saudi food and drug authority. Medical Device Labelling Requirements Saudi Arabia.
From www.greenlight.guru
Medical Device Labeling Definition & Requirements Medical Device Labelling Requirements Saudi Arabia To obtain mdma, a medical device shall comply with the applicable regulatory requirements outlined in. sfda requires compliance with the unique device identification (udi) regulations on all medical device. distributors of medical devices, healthcare providers importing medical devices, and any party who is involved in importing medical. the purpose of this document is to clarify labelling requirements. Medical Device Labelling Requirements Saudi Arabia.
From mavink.com
Medical Device Labeling Symbols Medical Device Labelling Requirements Saudi Arabia the purpose of this document is to clarify labelling requirements for medical devices such that they comply with the. sfda requires compliance with the unique device identification (udi) regulations on all medical device. distributors of medical devices, healthcare providers importing medical devices, and any party who is involved in importing medical. To obtain mdma, a medical device. Medical Device Labelling Requirements Saudi Arabia.
From www.techsollifesciences.com
EU MDR & IVDR Medical Device Labelling Requirements Medical Device Labelling Requirements Saudi Arabia the purpose of this document is to clarify labelling requirements for medical devices such that they comply with the. distributors of medical devices, healthcare providers importing medical devices, and any party who is involved in importing medical. sfda requires compliance with the unique device identification (udi) regulations on all medical device. To obtain mdma, a medical device. Medical Device Labelling Requirements Saudi Arabia.
From mungfali.com
Medical Device Labeling Symbols Medical Device Labelling Requirements Saudi Arabia To obtain mdma, a medical device shall comply with the applicable regulatory requirements outlined in. sfda requires compliance with the unique device identification (udi) regulations on all medical device. The saudi food and drug authority (sfda), the country’s regulating authority in the sphere of medical devices, has published guidance. the purpose of this document is to clarify labelling. Medical Device Labelling Requirements Saudi Arabia.
From www.meddeviceonline.com
Medical Device Labeling New ISO 152231 FDA Guidance UDI Medical Device Labelling Requirements Saudi Arabia distributors of medical devices, healthcare providers importing medical devices, and any party who is involved in importing medical. The saudi food and drug authority (sfda), the country’s regulating authority in the sphere of medical devices, has published guidance. To obtain mdma, a medical device shall comply with the applicable regulatory requirements outlined in. the purpose of this document. Medical Device Labelling Requirements Saudi Arabia.
From i-regulations.com
launching medical devices unique device identification Saudi DI Medical Device Labelling Requirements Saudi Arabia The saudi food and drug authority (sfda), the country’s regulating authority in the sphere of medical devices, has published guidance. To obtain mdma, a medical device shall comply with the applicable regulatory requirements outlined in. the purpose of this document is to clarify labelling requirements for medical devices such that they comply with the. sfda requires compliance with. Medical Device Labelling Requirements Saudi Arabia.
From mungfali.com
FDA Medical Device Label Symbols Medical Device Labelling Requirements Saudi Arabia distributors of medical devices, healthcare providers importing medical devices, and any party who is involved in importing medical. the purpose of this document is to clarify labelling requirements for medical devices such that they comply with the. sfda requires compliance with the unique device identification (udi) regulations on all medical device. To obtain mdma, a medical device. Medical Device Labelling Requirements Saudi Arabia.
From www.presentationeze.com
FDA Medical Device Labeling requirements. PresentationEZE Medical Device Labelling Requirements Saudi Arabia The saudi food and drug authority (sfda), the country’s regulating authority in the sphere of medical devices, has published guidance. distributors of medical devices, healthcare providers importing medical devices, and any party who is involved in importing medical. To obtain mdma, a medical device shall comply with the applicable regulatory requirements outlined in. the purpose of this document. Medical Device Labelling Requirements Saudi Arabia.
From www.doovi.com
15327_FDA Medical Device Regulations Labeling Require... Doovi Medical Device Labelling Requirements Saudi Arabia the purpose of this document is to clarify labelling requirements for medical devices such that they comply with the. To obtain mdma, a medical device shall comply with the applicable regulatory requirements outlined in. The saudi food and drug authority (sfda), the country’s regulating authority in the sphere of medical devices, has published guidance. distributors of medical devices,. Medical Device Labelling Requirements Saudi Arabia.
From pharmaknowl.com
SFDA Labelling Requirements PharmaKnowl Medical Device Labelling Requirements Saudi Arabia To obtain mdma, a medical device shall comply with the applicable regulatory requirements outlined in. the purpose of this document is to clarify labelling requirements for medical devices such that they comply with the. sfda requires compliance with the unique device identification (udi) regulations on all medical device. distributors of medical devices, healthcare providers importing medical devices,. Medical Device Labelling Requirements Saudi Arabia.
From www.londontranslations.co.uk
Medical device labelling What is it & what are the requirements? Medical Device Labelling Requirements Saudi Arabia The saudi food and drug authority (sfda), the country’s regulating authority in the sphere of medical devices, has published guidance. the purpose of this document is to clarify labelling requirements for medical devices such that they comply with the. To obtain mdma, a medical device shall comply with the applicable regulatory requirements outlined in. distributors of medical devices,. Medical Device Labelling Requirements Saudi Arabia.
From pharmaknowl.com
SFDA Medical Device Registration (MDMA 2024) PharmaKnowl Consulting Medical Device Labelling Requirements Saudi Arabia The saudi food and drug authority (sfda), the country’s regulating authority in the sphere of medical devices, has published guidance. distributors of medical devices, healthcare providers importing medical devices, and any party who is involved in importing medical. sfda requires compliance with the unique device identification (udi) regulations on all medical device. To obtain mdma, a medical device. Medical Device Labelling Requirements Saudi Arabia.
From medicaldevicelicense.com
EU MDR Medical Device Labeling RequirementsA Complete Guide Medical Device Labelling Requirements Saudi Arabia distributors of medical devices, healthcare providers importing medical devices, and any party who is involved in importing medical. The saudi food and drug authority (sfda), the country’s regulating authority in the sphere of medical devices, has published guidance. the purpose of this document is to clarify labelling requirements for medical devices such that they comply with the. To. Medical Device Labelling Requirements Saudi Arabia.
From www.regdesk.co
FDA Guidance on General Device Labeling RegDesk Medical Device Labelling Requirements Saudi Arabia the purpose of this document is to clarify labelling requirements for medical devices such that they comply with the. sfda requires compliance with the unique device identification (udi) regulations on all medical device. To obtain mdma, a medical device shall comply with the applicable regulatory requirements outlined in. distributors of medical devices, healthcare providers importing medical devices,. Medical Device Labelling Requirements Saudi Arabia.
From www.dasco.com
Medical Device Labels Dasco Medical Device Labelling Requirements Saudi Arabia distributors of medical devices, healthcare providers importing medical devices, and any party who is involved in importing medical. the purpose of this document is to clarify labelling requirements for medical devices such that they comply with the. The saudi food and drug authority (sfda), the country’s regulating authority in the sphere of medical devices, has published guidance. To. Medical Device Labelling Requirements Saudi Arabia.
From www.orielstat.com
Understanding FDA and EU Medical Device Labeling Requirements Oriel Medical Device Labelling Requirements Saudi Arabia sfda requires compliance with the unique device identification (udi) regulations on all medical device. To obtain mdma, a medical device shall comply with the applicable regulatory requirements outlined in. The saudi food and drug authority (sfda), the country’s regulating authority in the sphere of medical devices, has published guidance. distributors of medical devices, healthcare providers importing medical devices,. Medical Device Labelling Requirements Saudi Arabia.
From www.greenlight.guru
Am I Complying with FDA Medical Device Labeling Requirements? Medical Device Labelling Requirements Saudi Arabia The saudi food and drug authority (sfda), the country’s regulating authority in the sphere of medical devices, has published guidance. the purpose of this document is to clarify labelling requirements for medical devices such that they comply with the. distributors of medical devices, healthcare providers importing medical devices, and any party who is involved in importing medical. To. Medical Device Labelling Requirements Saudi Arabia.
From www.slideserve.com
PPT Medical Device Labeling PowerPoint Presentation, free download Medical Device Labelling Requirements Saudi Arabia sfda requires compliance with the unique device identification (udi) regulations on all medical device. To obtain mdma, a medical device shall comply with the applicable regulatory requirements outlined in. distributors of medical devices, healthcare providers importing medical devices, and any party who is involved in importing medical. The saudi food and drug authority (sfda), the country’s regulating authority. Medical Device Labelling Requirements Saudi Arabia.