Stability Testing Pull Window . A wide range of practices exist. The purpose of stability testing is to provide evidence of how the quality of an api or fpp varies with time under the influence of a variety of. I would like to know the time window used for the analysis of stability samples after withdrawl from the chambers? This document defines the stability data package for a new drug substance or drug product that is sufficient for a registration application within the. Stability study sop prepared according to ich guidelines with required stability study sample incubation, sample. Stability studies should include testing of those attributes of the drug substance that are susceptible to change during storage and are likely to. Stability testing of existing active ingredients and related finished products. A pull window is the range of time allowed before and after the scheduled calendar date for the withdrawing of samples for testing at a specific time point. Stability testing for applications for variations to marketing authorisation. This article will define the purpose of stability protocols, explore their potential elements, help us understand the impact of poorly designed protocols and learn how stability review boards can.
from www.mdpi.com
A pull window is the range of time allowed before and after the scheduled calendar date for the withdrawing of samples for testing at a specific time point. Stability studies should include testing of those attributes of the drug substance that are susceptible to change during storage and are likely to. The purpose of stability testing is to provide evidence of how the quality of an api or fpp varies with time under the influence of a variety of. This article will define the purpose of stability protocols, explore their potential elements, help us understand the impact of poorly designed protocols and learn how stability review boards can. Stability study sop prepared according to ich guidelines with required stability study sample incubation, sample. A wide range of practices exist. This document defines the stability data package for a new drug substance or drug product that is sufficient for a registration application within the. I would like to know the time window used for the analysis of stability samples after withdrawl from the chambers? Stability testing for applications for variations to marketing authorisation. Stability testing of existing active ingredients and related finished products.
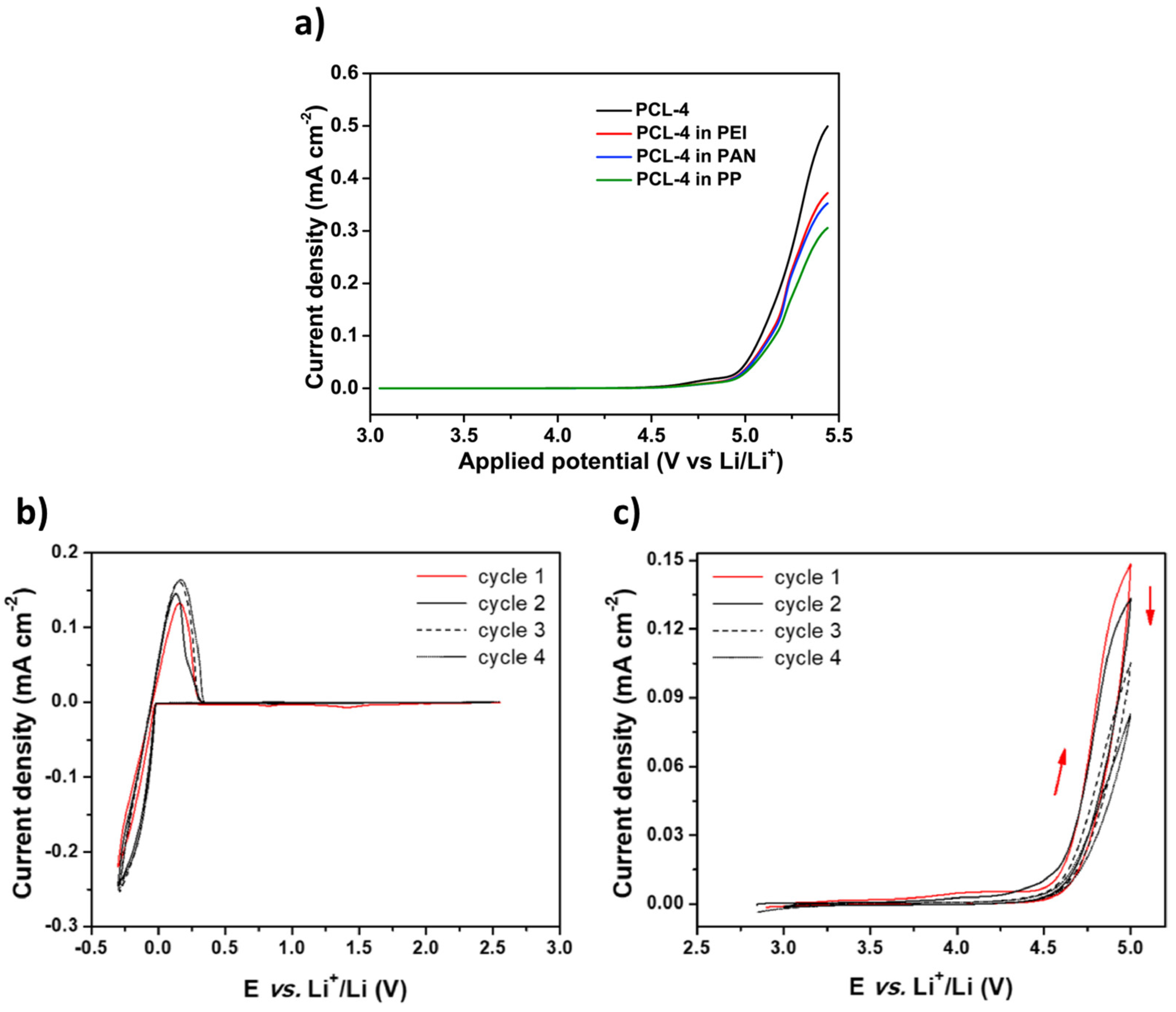
Materials Free FullText A Critical Review for an Accurate
Stability Testing Pull Window Stability testing for applications for variations to marketing authorisation. Stability testing for applications for variations to marketing authorisation. This article will define the purpose of stability protocols, explore their potential elements, help us understand the impact of poorly designed protocols and learn how stability review boards can. The purpose of stability testing is to provide evidence of how the quality of an api or fpp varies with time under the influence of a variety of. A pull window is the range of time allowed before and after the scheduled calendar date for the withdrawing of samples for testing at a specific time point. Stability study sop prepared according to ich guidelines with required stability study sample incubation, sample. Stability studies should include testing of those attributes of the drug substance that are susceptible to change during storage and are likely to. This document defines the stability data package for a new drug substance or drug product that is sufficient for a registration application within the. Stability testing of existing active ingredients and related finished products. I would like to know the time window used for the analysis of stability samples after withdrawl from the chambers? A wide range of practices exist.
From www.researchgate.net
Schematic representation of the electrolyte stability window with Stability Testing Pull Window The purpose of stability testing is to provide evidence of how the quality of an api or fpp varies with time under the influence of a variety of. Stability testing of existing active ingredients and related finished products. I would like to know the time window used for the analysis of stability samples after withdrawl from the chambers? Stability testing. Stability Testing Pull Window.
From www.researchgate.net
Stability test for the TPCL window. a) Stability test of the TPCL Stability Testing Pull Window A pull window is the range of time allowed before and after the scheduled calendar date for the withdrawing of samples for testing at a specific time point. A wide range of practices exist. Stability testing of existing active ingredients and related finished products. This document defines the stability data package for a new drug substance or drug product that. Stability Testing Pull Window.
From www.researchgate.net
The electrochemical stability window of electrolytes a Linear Stability Testing Pull Window Stability testing for applications for variations to marketing authorisation. A pull window is the range of time allowed before and after the scheduled calendar date for the withdrawing of samples for testing at a specific time point. The purpose of stability testing is to provide evidence of how the quality of an api or fpp varies with time under the. Stability Testing Pull Window.
From www.researchgate.net
The device of isometric pushpull strengths and the schematic testing Stability Testing Pull Window A pull window is the range of time allowed before and after the scheduled calendar date for the withdrawing of samples for testing at a specific time point. I would like to know the time window used for the analysis of stability samples after withdrawl from the chambers? This document defines the stability data package for a new drug substance. Stability Testing Pull Window.
From www.researchgate.net
Stability window of the parameters in the densitydependent quark mass Stability Testing Pull Window This article will define the purpose of stability protocols, explore their potential elements, help us understand the impact of poorly designed protocols and learn how stability review boards can. A pull window is the range of time allowed before and after the scheduled calendar date for the withdrawing of samples for testing at a specific time point. This document defines. Stability Testing Pull Window.
From www.youtube.com
Easy Window Pulls for Real Estate Photography YouTube Stability Testing Pull Window This document defines the stability data package for a new drug substance or drug product that is sufficient for a registration application within the. I would like to know the time window used for the analysis of stability samples after withdrawl from the chambers? This article will define the purpose of stability protocols, explore their potential elements, help us understand. Stability Testing Pull Window.
From ritmindustry.com
Stability test chamber / humidity / climatic / with window RITM Industry Stability Testing Pull Window Stability studies should include testing of those attributes of the drug substance that are susceptible to change during storage and are likely to. I would like to know the time window used for the analysis of stability samples after withdrawl from the chambers? A wide range of practices exist. This article will define the purpose of stability protocols, explore their. Stability Testing Pull Window.
From www.researchgate.net
Stability window determination a) anodic (I a ) and cathodic current Stability Testing Pull Window The purpose of stability testing is to provide evidence of how the quality of an api or fpp varies with time under the influence of a variety of. Stability testing of existing active ingredients and related finished products. Stability testing for applications for variations to marketing authorisation. A wide range of practices exist. This document defines the stability data package. Stability Testing Pull Window.
From www.researchgate.net
Stability window obtained with the MIT bag model with vector Stability Testing Pull Window A wide range of practices exist. I would like to know the time window used for the analysis of stability samples after withdrawl from the chambers? The purpose of stability testing is to provide evidence of how the quality of an api or fpp varies with time under the influence of a variety of. This article will define the purpose. Stability Testing Pull Window.
From www.chromatographyonline.com
Stability Studies and Testing of Pharmaceuticals An Overview Stability Testing Pull Window The purpose of stability testing is to provide evidence of how the quality of an api or fpp varies with time under the influence of a variety of. Stability studies should include testing of those attributes of the drug substance that are susceptible to change during storage and are likely to. A pull window is the range of time allowed. Stability Testing Pull Window.
From www.researchgate.net
A pullin and stability experiment showing pullin at 8 V and Stability Testing Pull Window Stability testing of existing active ingredients and related finished products. I would like to know the time window used for the analysis of stability samples after withdrawl from the chambers? Stability study sop prepared according to ich guidelines with required stability study sample incubation, sample. A wide range of practices exist. This document defines the stability data package for a. Stability Testing Pull Window.
From www.slideshare.net
Ich guideline for stability testing Stability Testing Pull Window A pull window is the range of time allowed before and after the scheduled calendar date for the withdrawing of samples for testing at a specific time point. Stability testing for applications for variations to marketing authorisation. Stability study sop prepared according to ich guidelines with required stability study sample incubation, sample. This document defines the stability data package for. Stability Testing Pull Window.
From www.researchgate.net
Rolling window analysis for model stability tests. Download Stability Testing Pull Window This document defines the stability data package for a new drug substance or drug product that is sufficient for a registration application within the. Stability testing for applications for variations to marketing authorisation. The purpose of stability testing is to provide evidence of how the quality of an api or fpp varies with time under the influence of a variety. Stability Testing Pull Window.
From www.slideshare.net
Ich guideline for stability testing Stability Testing Pull Window Stability testing of existing active ingredients and related finished products. This document defines the stability data package for a new drug substance or drug product that is sufficient for a registration application within the. A wide range of practices exist. A pull window is the range of time allowed before and after the scheduled calendar date for the withdrawing of. Stability Testing Pull Window.
From malwaretips.com
Remove Windows Stability Maximizer Uninstall Instructions Stability Testing Pull Window Stability testing of existing active ingredients and related finished products. This document defines the stability data package for a new drug substance or drug product that is sufficient for a registration application within the. The purpose of stability testing is to provide evidence of how the quality of an api or fpp varies with time under the influence of a. Stability Testing Pull Window.
From www.researchgate.net
The electrochemical stability window of the hybrid electrolyte on Stability Testing Pull Window Stability study sop prepared according to ich guidelines with required stability study sample incubation, sample. This document defines the stability data package for a new drug substance or drug product that is sufficient for a registration application within the. A pull window is the range of time allowed before and after the scheduled calendar date for the withdrawing of samples. Stability Testing Pull Window.
From www.mdpi.com
Pharmaceutics Free FullText LongTerm Stability Prediction for Stability Testing Pull Window Stability studies should include testing of those attributes of the drug substance that are susceptible to change during storage and are likely to. A wide range of practices exist. A pull window is the range of time allowed before and after the scheduled calendar date for the withdrawing of samples for testing at a specific time point. I would like. Stability Testing Pull Window.
From q1scientific.ie
A Q&A guide to stability storage Q1 Scientific Stability Testing Pull Window This article will define the purpose of stability protocols, explore their potential elements, help us understand the impact of poorly designed protocols and learn how stability review boards can. Stability testing for applications for variations to marketing authorisation. Stability study sop prepared according to ich guidelines with required stability study sample incubation, sample. I would like to know the time. Stability Testing Pull Window.
From www.researchgate.net
Electrochemical stability window of (a) LP40, (b) 1 M LiPF 6 dissolved Stability Testing Pull Window This document defines the stability data package for a new drug substance or drug product that is sufficient for a registration application within the. This article will define the purpose of stability protocols, explore their potential elements, help us understand the impact of poorly designed protocols and learn how stability review boards can. Stability testing for applications for variations to. Stability Testing Pull Window.
From malwaretips.com
Remove Windows Stability Maximizer Uninstall Instructions Stability Testing Pull Window A pull window is the range of time allowed before and after the scheduled calendar date for the withdrawing of samples for testing at a specific time point. This document defines the stability data package for a new drug substance or drug product that is sufficient for a registration application within the. A wide range of practices exist. The purpose. Stability Testing Pull Window.
From www.researchgate.net
Calculated stability window and products for (a) Na 3 PS Stability Testing Pull Window A pull window is the range of time allowed before and after the scheduled calendar date for the withdrawing of samples for testing at a specific time point. This article will define the purpose of stability protocols, explore their potential elements, help us understand the impact of poorly designed protocols and learn how stability review boards can. I would like. Stability Testing Pull Window.
From www.mlc-cad.com
Optimizing Windows to Maximize Stability & Performance Stability Testing Pull Window A wide range of practices exist. Stability testing of existing active ingredients and related finished products. This document defines the stability data package for a new drug substance or drug product that is sufficient for a registration application within the. I would like to know the time window used for the analysis of stability samples after withdrawl from the chambers?. Stability Testing Pull Window.
From mavink.com
Ftir Scan Of Water Stability Testing Pull Window The purpose of stability testing is to provide evidence of how the quality of an api or fpp varies with time under the influence of a variety of. This article will define the purpose of stability protocols, explore their potential elements, help us understand the impact of poorly designed protocols and learn how stability review boards can. A wide range. Stability Testing Pull Window.
From www.slideserve.com
PPT Stability Testing PowerPoint Presentation ID481423 Stability Testing Pull Window The purpose of stability testing is to provide evidence of how the quality of an api or fpp varies with time under the influence of a variety of. This document defines the stability data package for a new drug substance or drug product that is sufficient for a registration application within the. Stability testing for applications for variations to marketing. Stability Testing Pull Window.
From www.researchgate.net
The electrochemical stability window of the PEI based separator and the Stability Testing Pull Window A wide range of practices exist. Stability testing for applications for variations to marketing authorisation. A pull window is the range of time allowed before and after the scheduled calendar date for the withdrawing of samples for testing at a specific time point. Stability study sop prepared according to ich guidelines with required stability study sample incubation, sample. This document. Stability Testing Pull Window.
From 4sysops.com
Monitoring Windows system stability with PowerShell 4sysops Stability Testing Pull Window The purpose of stability testing is to provide evidence of how the quality of an api or fpp varies with time under the influence of a variety of. This document defines the stability data package for a new drug substance or drug product that is sufficient for a registration application within the. Stability study sop prepared according to ich guidelines. Stability Testing Pull Window.
From www.researchgate.net
Timeevolution of stability window with physicochemical effects Stability Testing Pull Window This article will define the purpose of stability protocols, explore their potential elements, help us understand the impact of poorly designed protocols and learn how stability review boards can. A wide range of practices exist. Stability testing for applications for variations to marketing authorisation. This document defines the stability data package for a new drug substance or drug product that. Stability Testing Pull Window.
From www.researchgate.net
The visual stability intervals window Download Scientific Diagram Stability Testing Pull Window This article will define the purpose of stability protocols, explore their potential elements, help us understand the impact of poorly designed protocols and learn how stability review boards can. Stability testing for applications for variations to marketing authorisation. A wide range of practices exist. The purpose of stability testing is to provide evidence of how the quality of an api. Stability Testing Pull Window.
From uby-testequipment.en.made-in-china.com
up6120 Observation Window with Operating Hole, Climate Stability Room Stability Testing Pull Window This article will define the purpose of stability protocols, explore their potential elements, help us understand the impact of poorly designed protocols and learn how stability review boards can. A wide range of practices exist. Stability testing for applications for variations to marketing authorisation. Stability testing of existing active ingredients and related finished products. Stability study sop prepared according to. Stability Testing Pull Window.
From www.researchgate.net
Stability window obtained with the MIT bag model with vector Stability Testing Pull Window Stability testing of existing active ingredients and related finished products. A wide range of practices exist. This article will define the purpose of stability protocols, explore their potential elements, help us understand the impact of poorly designed protocols and learn how stability review boards can. This document defines the stability data package for a new drug substance or drug product. Stability Testing Pull Window.
From www.researchgate.net
"List of stability determination paremeters" window. Download Stability Testing Pull Window A wide range of practices exist. Stability studies should include testing of those attributes of the drug substance that are susceptible to change during storage and are likely to. Stability study sop prepared according to ich guidelines with required stability study sample incubation, sample. The purpose of stability testing is to provide evidence of how the quality of an api. Stability Testing Pull Window.
From www.youtube.com
Pull Test Retropulsion Test Postural Stability Test For Parkinson's Stability Testing Pull Window Stability study sop prepared according to ich guidelines with required stability study sample incubation, sample. A wide range of practices exist. This article will define the purpose of stability protocols, explore their potential elements, help us understand the impact of poorly designed protocols and learn how stability review boards can. This document defines the stability data package for a new. Stability Testing Pull Window.
From www.mdpi.com
Materials Free FullText A Critical Review for an Accurate Stability Testing Pull Window A pull window is the range of time allowed before and after the scheduled calendar date for the withdrawing of samples for testing at a specific time point. This document defines the stability data package for a new drug substance or drug product that is sufficient for a registration application within the. Stability testing for applications for variations to marketing. Stability Testing Pull Window.
From www.researchgate.net
(a) Electrochemical stability window (ESW) of different electrolytes Stability Testing Pull Window Stability testing for applications for variations to marketing authorisation. The purpose of stability testing is to provide evidence of how the quality of an api or fpp varies with time under the influence of a variety of. I would like to know the time window used for the analysis of stability samples after withdrawl from the chambers? Stability study sop. Stability Testing Pull Window.
From www.youtube.com
Simple Window Pulls for Real Estate Photography... Remove Glare Stability Testing Pull Window Stability testing for applications for variations to marketing authorisation. I would like to know the time window used for the analysis of stability samples after withdrawl from the chambers? The purpose of stability testing is to provide evidence of how the quality of an api or fpp varies with time under the influence of a variety of. Stability testing of. Stability Testing Pull Window.