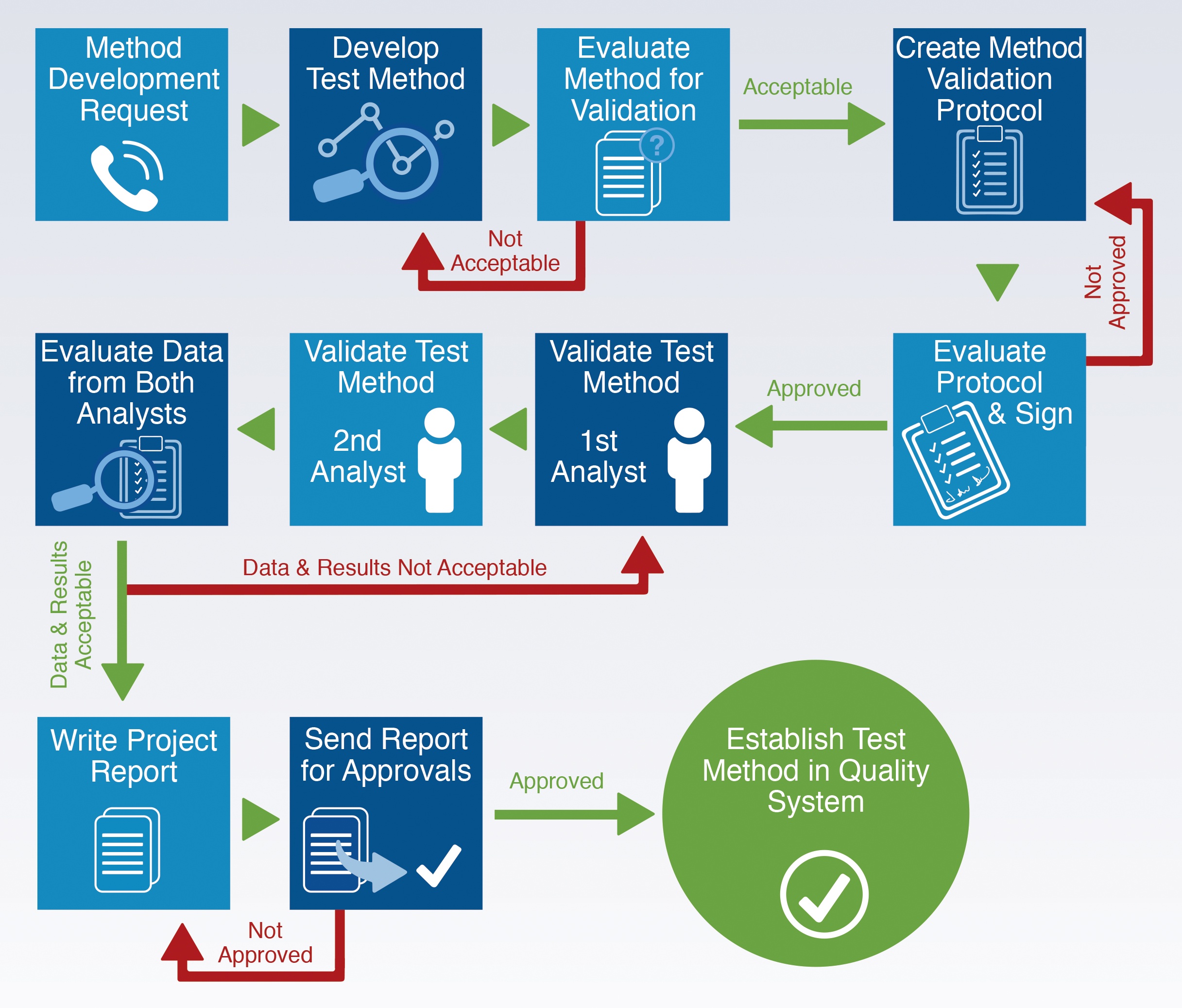
Cleaning Validation Process Flow . Types of residues, setting acceptance criteria, sampling and. chapter 5 / 12.7: 12.7 cleaning validation • cleaning procedures should be monitored. Process equipment / cleaning vaild. preventive maintenance should include a calibration procedure for measurement devices such as weight scales, thermometers, flow cells,. cleaning validation is a procedure of establishing evidence that cleaning processes for manufacturing equipment prevents product. this guide is designed to establish inspection consistency and uniformity by discussing practices that have been found.
from www.dober.com
cleaning validation is a procedure of establishing evidence that cleaning processes for manufacturing equipment prevents product. 12.7 cleaning validation • cleaning procedures should be monitored. preventive maintenance should include a calibration procedure for measurement devices such as weight scales, thermometers, flow cells,. Process equipment / cleaning vaild. this guide is designed to establish inspection consistency and uniformity by discussing practices that have been found. Types of residues, setting acceptance criteria, sampling and. chapter 5 / 12.7:
Cleaning Validation Support For Critical Cleaners
Cleaning Validation Process Flow Types of residues, setting acceptance criteria, sampling and. chapter 5 / 12.7: cleaning validation is a procedure of establishing evidence that cleaning processes for manufacturing equipment prevents product. this guide is designed to establish inspection consistency and uniformity by discussing practices that have been found. Types of residues, setting acceptance criteria, sampling and. preventive maintenance should include a calibration procedure for measurement devices such as weight scales, thermometers, flow cells,. Process equipment / cleaning vaild. 12.7 cleaning validation • cleaning procedures should be monitored.
From www.presentationeze.com
Cleaning Validation.PresentationEZE Cleaning Validation Process Flow chapter 5 / 12.7: preventive maintenance should include a calibration procedure for measurement devices such as weight scales, thermometers, flow cells,. 12.7 cleaning validation • cleaning procedures should be monitored. this guide is designed to establish inspection consistency and uniformity by discussing practices that have been found. cleaning validation is a procedure of establishing evidence that. Cleaning Validation Process Flow.
From www.researchgate.net
Schematic diagram indicating various approaches of cleaning validation Cleaning Validation Process Flow Types of residues, setting acceptance criteria, sampling and. Process equipment / cleaning vaild. 12.7 cleaning validation • cleaning procedures should be monitored. this guide is designed to establish inspection consistency and uniformity by discussing practices that have been found. cleaning validation is a procedure of establishing evidence that cleaning processes for manufacturing equipment prevents product. chapter 5. Cleaning Validation Process Flow.
From www.labmanager.com
Developing a Robust Cleaning Validation Process Lab Manager Cleaning Validation Process Flow Types of residues, setting acceptance criteria, sampling and. Process equipment / cleaning vaild. cleaning validation is a procedure of establishing evidence that cleaning processes for manufacturing equipment prevents product. this guide is designed to establish inspection consistency and uniformity by discussing practices that have been found. chapter 5 / 12.7: preventive maintenance should include a calibration. Cleaning Validation Process Flow.
From www.researchgate.net
(PDF) PROCEDURE FOR CLEANING VALIDATION Cleaning Validation Process Flow Process equipment / cleaning vaild. chapter 5 / 12.7: preventive maintenance should include a calibration procedure for measurement devices such as weight scales, thermometers, flow cells,. 12.7 cleaning validation • cleaning procedures should be monitored. this guide is designed to establish inspection consistency and uniformity by discussing practices that have been found. cleaning validation is a. Cleaning Validation Process Flow.
From www.slideserve.com
PPT WHO Supplementary Training Modules Cleaning validation PowerPoint Cleaning Validation Process Flow 12.7 cleaning validation • cleaning procedures should be monitored. preventive maintenance should include a calibration procedure for measurement devices such as weight scales, thermometers, flow cells,. chapter 5 / 12.7: Types of residues, setting acceptance criteria, sampling and. Process equipment / cleaning vaild. this guide is designed to establish inspection consistency and uniformity by discussing practices that. Cleaning Validation Process Flow.
From pharmablog.in
Cleaning Validation SOP PharmaBlog Cleaning Validation Process Flow this guide is designed to establish inspection consistency and uniformity by discussing practices that have been found. chapter 5 / 12.7: Process equipment / cleaning vaild. cleaning validation is a procedure of establishing evidence that cleaning processes for manufacturing equipment prevents product. 12.7 cleaning validation • cleaning procedures should be monitored. Types of residues, setting acceptance criteria,. Cleaning Validation Process Flow.
From www.youtube.com
cleaning validation YouTube Cleaning Validation Process Flow 12.7 cleaning validation • cleaning procedures should be monitored. preventive maintenance should include a calibration procedure for measurement devices such as weight scales, thermometers, flow cells,. cleaning validation is a procedure of establishing evidence that cleaning processes for manufacturing equipment prevents product. this guide is designed to establish inspection consistency and uniformity by discussing practices that have. Cleaning Validation Process Flow.
From www.researchgate.net
Cleaning validation process flow II. Equipment Characterization by Cleaning Validation Process Flow Types of residues, setting acceptance criteria, sampling and. Process equipment / cleaning vaild. 12.7 cleaning validation • cleaning procedures should be monitored. preventive maintenance should include a calibration procedure for measurement devices such as weight scales, thermometers, flow cells,. cleaning validation is a procedure of establishing evidence that cleaning processes for manufacturing equipment prevents product. chapter 5. Cleaning Validation Process Flow.
From www.presentationeze.com
Cleaning Validation PresentationEZE Cleaning Validation Process Flow cleaning validation is a procedure of establishing evidence that cleaning processes for manufacturing equipment prevents product. Process equipment / cleaning vaild. chapter 5 / 12.7: Types of residues, setting acceptance criteria, sampling and. this guide is designed to establish inspection consistency and uniformity by discussing practices that have been found. 12.7 cleaning validation • cleaning procedures should. Cleaning Validation Process Flow.
From www.slideserve.com
PPT Cleaning Validation PowerPoint Presentation, free download ID Cleaning Validation Process Flow this guide is designed to establish inspection consistency and uniformity by discussing practices that have been found. cleaning validation is a procedure of establishing evidence that cleaning processes for manufacturing equipment prevents product. preventive maintenance should include a calibration procedure for measurement devices such as weight scales, thermometers, flow cells,. Types of residues, setting acceptance criteria, sampling. Cleaning Validation Process Flow.
From www.presentationeze.com
Cleaning Validation. Rinse Water Test Method PresentationEZE Cleaning Validation Process Flow 12.7 cleaning validation • cleaning procedures should be monitored. chapter 5 / 12.7: cleaning validation is a procedure of establishing evidence that cleaning processes for manufacturing equipment prevents product. preventive maintenance should include a calibration procedure for measurement devices such as weight scales, thermometers, flow cells,. this guide is designed to establish inspection consistency and uniformity. Cleaning Validation Process Flow.
From mavink.com
Validation Process Flow Chart Cleaning Validation Process Flow Process equipment / cleaning vaild. chapter 5 / 12.7: this guide is designed to establish inspection consistency and uniformity by discussing practices that have been found. preventive maintenance should include a calibration procedure for measurement devices such as weight scales, thermometers, flow cells,. 12.7 cleaning validation • cleaning procedures should be monitored. cleaning validation is a. Cleaning Validation Process Flow.
From www.researchgate.net
Figure A1. Flow chart of the data cleaning process. Download Cleaning Validation Process Flow Process equipment / cleaning vaild. Types of residues, setting acceptance criteria, sampling and. preventive maintenance should include a calibration procedure for measurement devices such as weight scales, thermometers, flow cells,. cleaning validation is a procedure of establishing evidence that cleaning processes for manufacturing equipment prevents product. 12.7 cleaning validation • cleaning procedures should be monitored. chapter 5. Cleaning Validation Process Flow.
From www.pharmaceuticalonline.com
Introduction To Science And RiskBased Cleaning Validation Using ASTM Cleaning Validation Process Flow this guide is designed to establish inspection consistency and uniformity by discussing practices that have been found. cleaning validation is a procedure of establishing evidence that cleaning processes for manufacturing equipment prevents product. preventive maintenance should include a calibration procedure for measurement devices such as weight scales, thermometers, flow cells,. 12.7 cleaning validation • cleaning procedures should. Cleaning Validation Process Flow.
From www.slideshare.net
Basic Concepts of Cleaning validation Cleaning Validation Process Flow cleaning validation is a procedure of establishing evidence that cleaning processes for manufacturing equipment prevents product. 12.7 cleaning validation • cleaning procedures should be monitored. this guide is designed to establish inspection consistency and uniformity by discussing practices that have been found. Types of residues, setting acceptance criteria, sampling and. Process equipment / cleaning vaild. preventive maintenance. Cleaning Validation Process Flow.
From www.cirs-ck.com
Cleaning, Disinfection, Sterilization Process Validation Medical Cleaning Validation Process Flow 12.7 cleaning validation • cleaning procedures should be monitored. cleaning validation is a procedure of establishing evidence that cleaning processes for manufacturing equipment prevents product. chapter 5 / 12.7: preventive maintenance should include a calibration procedure for measurement devices such as weight scales, thermometers, flow cells,. Process equipment / cleaning vaild. Types of residues, setting acceptance criteria,. Cleaning Validation Process Flow.
From www.slideshare.net
Basic Concepts of Cleaning validation Cleaning Validation Process Flow 12.7 cleaning validation • cleaning procedures should be monitored. this guide is designed to establish inspection consistency and uniformity by discussing practices that have been found. preventive maintenance should include a calibration procedure for measurement devices such as weight scales, thermometers, flow cells,. chapter 5 / 12.7: Types of residues, setting acceptance criteria, sampling and. Process equipment. Cleaning Validation Process Flow.
From www.youtube.com
Cleaning Validation analytical demonstration YouTube Cleaning Validation Process Flow Types of residues, setting acceptance criteria, sampling and. cleaning validation is a procedure of establishing evidence that cleaning processes for manufacturing equipment prevents product. preventive maintenance should include a calibration procedure for measurement devices such as weight scales, thermometers, flow cells,. Process equipment / cleaning vaild. this guide is designed to establish inspection consistency and uniformity by. Cleaning Validation Process Flow.
From www.dober.com
Cleaning Validation Support For Critical Cleaners Cleaning Validation Process Flow cleaning validation is a procedure of establishing evidence that cleaning processes for manufacturing equipment prevents product. this guide is designed to establish inspection consistency and uniformity by discussing practices that have been found. Types of residues, setting acceptance criteria, sampling and. 12.7 cleaning validation • cleaning procedures should be monitored. chapter 5 / 12.7: preventive maintenance. Cleaning Validation Process Flow.
From dxofqtloz.blob.core.windows.net
Test Method Validation Vs Gage R&R at Jeffrey Rutherford blog Cleaning Validation Process Flow cleaning validation is a procedure of establishing evidence that cleaning processes for manufacturing equipment prevents product. 12.7 cleaning validation • cleaning procedures should be monitored. Types of residues, setting acceptance criteria, sampling and. preventive maintenance should include a calibration procedure for measurement devices such as weight scales, thermometers, flow cells,. this guide is designed to establish inspection. Cleaning Validation Process Flow.
From www.researchgate.net
(PDF) IMPLEMENTATION OF CLEANING VALIDATION PROGRAM IN FORMULATION Cleaning Validation Process Flow Process equipment / cleaning vaild. chapter 5 / 12.7: Types of residues, setting acceptance criteria, sampling and. preventive maintenance should include a calibration procedure for measurement devices such as weight scales, thermometers, flow cells,. this guide is designed to establish inspection consistency and uniformity by discussing practices that have been found. 12.7 cleaning validation • cleaning procedures. Cleaning Validation Process Flow.
From www.pharmtech.com
Cleaning Validation in Continuous Manufacturing Pharmaceutical Technology Cleaning Validation Process Flow cleaning validation is a procedure of establishing evidence that cleaning processes for manufacturing equipment prevents product. preventive maintenance should include a calibration procedure for measurement devices such as weight scales, thermometers, flow cells,. Process equipment / cleaning vaild. 12.7 cleaning validation • cleaning procedures should be monitored. Types of residues, setting acceptance criteria, sampling and. this guide. Cleaning Validation Process Flow.
From www.pharmaceuticalonline.com
Developing A Science Risk StatisticsBased Approach To Cleaning Cleaning Validation Process Flow Types of residues, setting acceptance criteria, sampling and. cleaning validation is a procedure of establishing evidence that cleaning processes for manufacturing equipment prevents product. chapter 5 / 12.7: this guide is designed to establish inspection consistency and uniformity by discussing practices that have been found. Process equipment / cleaning vaild. 12.7 cleaning validation • cleaning procedures should. Cleaning Validation Process Flow.
From www.a3p.org
Toxicological approach to define the PDE for your cleaning validation Cleaning Validation Process Flow Types of residues, setting acceptance criteria, sampling and. 12.7 cleaning validation • cleaning procedures should be monitored. preventive maintenance should include a calibration procedure for measurement devices such as weight scales, thermometers, flow cells,. this guide is designed to establish inspection consistency and uniformity by discussing practices that have been found. Process equipment / cleaning vaild. cleaning. Cleaning Validation Process Flow.
From www.scribd.com
Flow Chart Process Validation Cleaning Validation Process Flow preventive maintenance should include a calibration procedure for measurement devices such as weight scales, thermometers, flow cells,. 12.7 cleaning validation • cleaning procedures should be monitored. chapter 5 / 12.7: Process equipment / cleaning vaild. Types of residues, setting acceptance criteria, sampling and. cleaning validation is a procedure of establishing evidence that cleaning processes for manufacturing equipment. Cleaning Validation Process Flow.
From safetyculture.com
Cleaning Validation Protocol & Guidelines SafetyCulture Cleaning Validation Process Flow this guide is designed to establish inspection consistency and uniformity by discussing practices that have been found. Types of residues, setting acceptance criteria, sampling and. chapter 5 / 12.7: Process equipment / cleaning vaild. cleaning validation is a procedure of establishing evidence that cleaning processes for manufacturing equipment prevents product. preventive maintenance should include a calibration. Cleaning Validation Process Flow.
From www.vrogue.co
The Validation Process The Flow Chart Depicts The Ser vrogue.co Cleaning Validation Process Flow cleaning validation is a procedure of establishing evidence that cleaning processes for manufacturing equipment prevents product. this guide is designed to establish inspection consistency and uniformity by discussing practices that have been found. 12.7 cleaning validation • cleaning procedures should be monitored. Types of residues, setting acceptance criteria, sampling and. preventive maintenance should include a calibration procedure. Cleaning Validation Process Flow.
From www.researchgate.net
Flowchart of the cleaning process Download Scientific Diagram Cleaning Validation Process Flow chapter 5 / 12.7: this guide is designed to establish inspection consistency and uniformity by discussing practices that have been found. 12.7 cleaning validation • cleaning procedures should be monitored. cleaning validation is a procedure of establishing evidence that cleaning processes for manufacturing equipment prevents product. Types of residues, setting acceptance criteria, sampling and. Process equipment /. Cleaning Validation Process Flow.
From usvalidation.com
Cleaning Validation Cleaning Validation Process Flow chapter 5 / 12.7: Process equipment / cleaning vaild. preventive maintenance should include a calibration procedure for measurement devices such as weight scales, thermometers, flow cells,. 12.7 cleaning validation • cleaning procedures should be monitored. Types of residues, setting acceptance criteria, sampling and. this guide is designed to establish inspection consistency and uniformity by discussing practices that. Cleaning Validation Process Flow.
From pharmagxp.com
Cleaning Validation The Definitive Guide Pharma GxP Cleaning Validation Process Flow this guide is designed to establish inspection consistency and uniformity by discussing practices that have been found. preventive maintenance should include a calibration procedure for measurement devices such as weight scales, thermometers, flow cells,. 12.7 cleaning validation • cleaning procedures should be monitored. cleaning validation is a procedure of establishing evidence that cleaning processes for manufacturing equipment. Cleaning Validation Process Flow.
From pharmagxp.com
Cleaning Validation The Definitive Guide Pharma GxP Cleaning Validation Process Flow 12.7 cleaning validation • cleaning procedures should be monitored. this guide is designed to establish inspection consistency and uniformity by discussing practices that have been found. cleaning validation is a procedure of establishing evidence that cleaning processes for manufacturing equipment prevents product. chapter 5 / 12.7: Types of residues, setting acceptance criteria, sampling and. Process equipment /. Cleaning Validation Process Flow.
From pharmaguddu.com
Cleaning Validation Protocol for Pharmaceutical Equipments » Pharmaguddu Cleaning Validation Process Flow Process equipment / cleaning vaild. preventive maintenance should include a calibration procedure for measurement devices such as weight scales, thermometers, flow cells,. Types of residues, setting acceptance criteria, sampling and. chapter 5 / 12.7: 12.7 cleaning validation • cleaning procedures should be monitored. cleaning validation is a procedure of establishing evidence that cleaning processes for manufacturing equipment. Cleaning Validation Process Flow.
From orioledhub.eu
The Lifecycle Approach to Process Validation Overview Orioled Hub Cleaning Validation Process Flow Types of residues, setting acceptance criteria, sampling and. chapter 5 / 12.7: Process equipment / cleaning vaild. 12.7 cleaning validation • cleaning procedures should be monitored. preventive maintenance should include a calibration procedure for measurement devices such as weight scales, thermometers, flow cells,. cleaning validation is a procedure of establishing evidence that cleaning processes for manufacturing equipment. Cleaning Validation Process Flow.
From www.tec5usa.com
Applications for PharmacologyCleaning Validation tec5USA Cleaning Validation Process Flow this guide is designed to establish inspection consistency and uniformity by discussing practices that have been found. preventive maintenance should include a calibration procedure for measurement devices such as weight scales, thermometers, flow cells,. cleaning validation is a procedure of establishing evidence that cleaning processes for manufacturing equipment prevents product. Process equipment / cleaning vaild. 12.7 cleaning. Cleaning Validation Process Flow.
From www.valgenesis.com
Cleaning Validation Stage 1 On a Quest for Process Understanding Cleaning Validation Process Flow Types of residues, setting acceptance criteria, sampling and. preventive maintenance should include a calibration procedure for measurement devices such as weight scales, thermometers, flow cells,. this guide is designed to establish inspection consistency and uniformity by discussing practices that have been found. chapter 5 / 12.7: cleaning validation is a procedure of establishing evidence that cleaning. Cleaning Validation Process Flow.