Titration Calculate Mole Ratio . Take known volume of known solution “a” and multiply it by molarity of solution a to calculate the moles of a. A titration calculation is a simple formula used to work out the concentration (in moles) of one of the reactants in a titration using the concentration of the other. From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that. First determine the moles of \(\ce{naoh}\) in the reaction. Next, apply the mole ratio calculation to determine the moles of. From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted. A write the balanced chemical equation for the reaction and calculate the number of moles of base needed to neutralize the ascorbic acid. B using mole ratios, determine. The coefficients of the substances in a balanced. Multiply the molarity of the strong base naoh by the volume of. At ph 7, the concentration of h₃o⁺ ion and oh⁻ ion is 1:1 (titration equivalence point). First determine the moles of \(\ce{naoh}\) in the reaction. 2h₂o ⇌ oh⁻ + h₃o⁺. A molar ratio is the proportion of moles of one substance to the moles of another substance in a chemical reaction.
from www.chegg.com
The coefficients of the substances in a balanced. Take known volume of known solution “a” and multiply it by molarity of solution a to calculate the moles of a. Next, apply the mole ratio calculation to determine the moles of. From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted. B using mole ratios, determine. A titration calculation is a simple formula used to work out the concentration (in moles) of one of the reactants in a titration using the concentration of the other. At ph 7, the concentration of h₃o⁺ ion and oh⁻ ion is 1:1 (titration equivalence point). 2h₂o ⇌ oh⁻ + h₃o⁺. A molar ratio is the proportion of moles of one substance to the moles of another substance in a chemical reaction. First determine the moles of \(\ce{naoh}\) in the reaction.
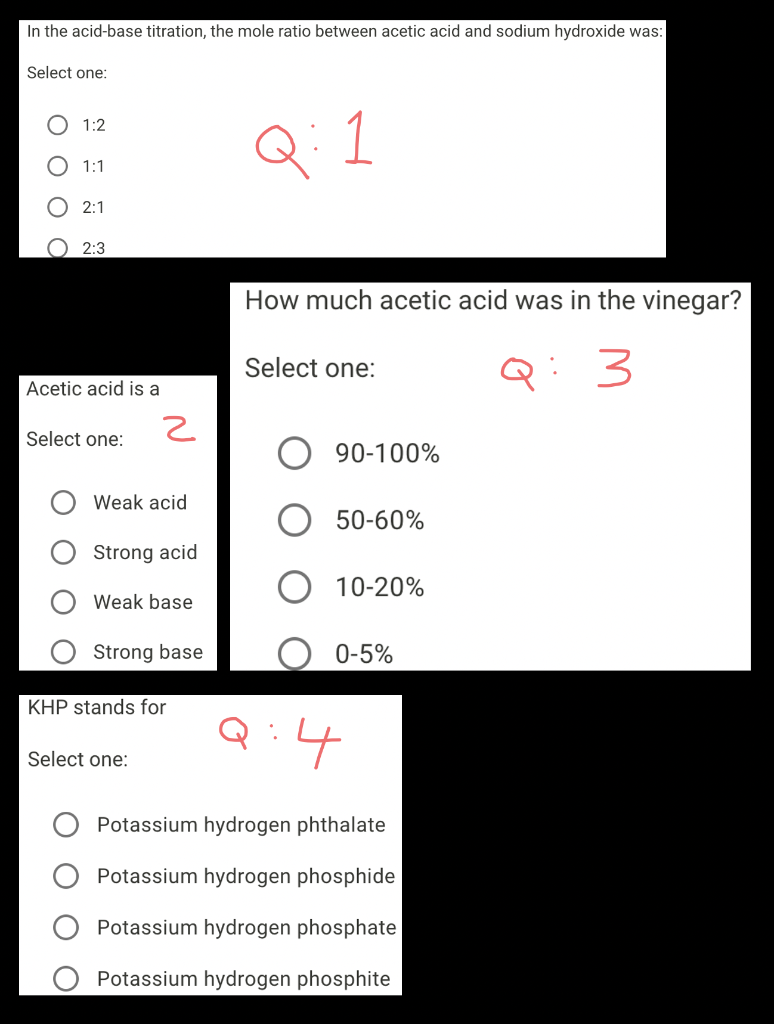
Solved In the acidbase titration, the mole ratio between
Titration Calculate Mole Ratio Take known volume of known solution “a” and multiply it by molarity of solution a to calculate the moles of a. The coefficients of the substances in a balanced. First determine the moles of \(\ce{naoh}\) in the reaction. From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted. First determine the moles of \(\ce{naoh}\) in the reaction. Next, apply the mole ratio calculation to determine the moles of. From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that. A molar ratio is the proportion of moles of one substance to the moles of another substance in a chemical reaction. At ph 7, the concentration of h₃o⁺ ion and oh⁻ ion is 1:1 (titration equivalence point). A write the balanced chemical equation for the reaction and calculate the number of moles of base needed to neutralize the ascorbic acid. A titration calculation is a simple formula used to work out the concentration (in moles) of one of the reactants in a titration using the concentration of the other. 2h₂o ⇌ oh⁻ + h₃o⁺. B using mole ratios, determine. Take known volume of known solution “a” and multiply it by molarity of solution a to calculate the moles of a. Multiply the molarity of the strong base naoh by the volume of.
From www.researchgate.net
Titration curves for CL (C140). a) pH as a function of mole ratio r{H Titration Calculate Mole Ratio Next, apply the mole ratio calculation to determine the moles of. A write the balanced chemical equation for the reaction and calculate the number of moles of base needed to neutralize the ascorbic acid. A titration calculation is a simple formula used to work out the concentration (in moles) of one of the reactants in a titration using the concentration. Titration Calculate Mole Ratio.
From celhcddi.blob.core.windows.net
Titration Lab Math at Mike Brown blog Titration Calculate Mole Ratio From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted. B using mole ratios, determine. 2h₂o ⇌ oh⁻ + h₃o⁺. Multiply the molarity of the strong base naoh by the volume of. Take known volume of known solution “a” and multiply it by molarity of solution a to calculate the moles of a. A write the balanced chemical equation. Titration Calculate Mole Ratio.
From www.slideserve.com
PPT Titration PowerPoint Presentation, free download ID3459790 Titration Calculate Mole Ratio First determine the moles of \(\ce{naoh}\) in the reaction. From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted. A molar ratio is the proportion of moles of one substance to the moles of another substance in a chemical reaction. Multiply the molarity of the strong base naoh by the volume of. A titration calculation is a simple formula. Titration Calculate Mole Ratio.
From www.youtube.com
Calculations Moles Titration Example1 YouTube Titration Calculate Mole Ratio First determine the moles of \(\ce{naoh}\) in the reaction. Next, apply the mole ratio calculation to determine the moles of. From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted. B using mole ratios, determine. From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that. A molar ratio is the proportion of moles of one substance to the moles. Titration Calculate Mole Ratio.
From www.youtube.com
KAC31.6 Titrations II (Redox) Finding Mole Ratio (Stoichiometry Titration Calculate Mole Ratio 2h₂o ⇌ oh⁻ + h₃o⁺. From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted. Take known volume of known solution “a” and multiply it by molarity of solution a to calculate the moles of a. The coefficients of the substances in a balanced. Multiply the molarity of the strong base naoh by the volume of. First determine the. Titration Calculate Mole Ratio.
From www.chegg.com
Solved (a) A Titration Of 5.00 ML Acetic Acid Required 14... Titration Calculate Mole Ratio A titration calculation is a simple formula used to work out the concentration (in moles) of one of the reactants in a titration using the concentration of the other. A molar ratio is the proportion of moles of one substance to the moles of another substance in a chemical reaction. Multiply the molarity of the strong base naoh by the. Titration Calculate Mole Ratio.
From www.numerade.com
SOLVED 03 Phenolphathalein Titratio Molesof HCLused in the titration Titration Calculate Mole Ratio 2h₂o ⇌ oh⁻ + h₃o⁺. The coefficients of the substances in a balanced. A titration calculation is a simple formula used to work out the concentration (in moles) of one of the reactants in a titration using the concentration of the other. First determine the moles of \(\ce{naoh}\) in the reaction. B using mole ratios, determine. From the mole ratio,. Titration Calculate Mole Ratio.
From www.youtube.com
Solving AcidBase Titration Problems YouTube Titration Calculate Mole Ratio A write the balanced chemical equation for the reaction and calculate the number of moles of base needed to neutralize the ascorbic acid. First determine the moles of \(\ce{naoh}\) in the reaction. Take known volume of known solution “a” and multiply it by molarity of solution a to calculate the moles of a. At ph 7, the concentration of h₃o⁺. Titration Calculate Mole Ratio.
From www.numerade.com
SOLVED From this mole value of NaOH, obtain the moles of CH3COOH in Titration Calculate Mole Ratio The coefficients of the substances in a balanced. A write the balanced chemical equation for the reaction and calculate the number of moles of base needed to neutralize the ascorbic acid. 2h₂o ⇌ oh⁻ + h₃o⁺. A titration calculation is a simple formula used to work out the concentration (in moles) of one of the reactants in a titration using. Titration Calculate Mole Ratio.
From www.slideserve.com
PPT TITRATION PowerPoint Presentation, free download ID1459481 Titration Calculate Mole Ratio At ph 7, the concentration of h₃o⁺ ion and oh⁻ ion is 1:1 (titration equivalence point). Take known volume of known solution “a” and multiply it by molarity of solution a to calculate the moles of a. Multiply the molarity of the strong base naoh by the volume of. First determine the moles of \(\ce{naoh}\) in the reaction. A write. Titration Calculate Mole Ratio.
From slideplayer.com
Acid Base Titrations Lesson ppt download Titration Calculate Mole Ratio A write the balanced chemical equation for the reaction and calculate the number of moles of base needed to neutralize the ascorbic acid. First determine the moles of \(\ce{naoh}\) in the reaction. From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that. The coefficients of the substances in a balanced. 2h₂o ⇌ oh⁻ + h₃o⁺. From the mole ratio, calculate. Titration Calculate Mole Ratio.
From www.wikihow.com
4 Ways to Calculate Molarity wikiHow Titration Calculate Mole Ratio Next, apply the mole ratio calculation to determine the moles of. A write the balanced chemical equation for the reaction and calculate the number of moles of base needed to neutralize the ascorbic acid. Multiply the molarity of the strong base naoh by the volume of. From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that. First determine the moles. Titration Calculate Mole Ratio.
From dxolxludj.blob.core.windows.net
Titration Calculating Moles at Gary Mosley blog Titration Calculate Mole Ratio First determine the moles of \(\ce{naoh}\) in the reaction. From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted. Take known volume of known solution “a” and multiply it by molarity of solution a to calculate the moles of a. From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that. Multiply the molarity of the strong base naoh by. Titration Calculate Mole Ratio.
From www.youtube.com
Molarity Chemistry Tutorial YouTube Titration Calculate Mole Ratio First determine the moles of \(\ce{naoh}\) in the reaction. A molar ratio is the proportion of moles of one substance to the moles of another substance in a chemical reaction. First determine the moles of \(\ce{naoh}\) in the reaction. A write the balanced chemical equation for the reaction and calculate the number of moles of base needed to neutralize the. Titration Calculate Mole Ratio.
From www.slideserve.com
PPT TITRATION PowerPoint Presentation, free download ID1459481 Titration Calculate Mole Ratio The coefficients of the substances in a balanced. 2h₂o ⇌ oh⁻ + h₃o⁺. A titration calculation is a simple formula used to work out the concentration (in moles) of one of the reactants in a titration using the concentration of the other. Next, apply the mole ratio calculation to determine the moles of. Take known volume of known solution “a”. Titration Calculate Mole Ratio.
From www.nagwa.com
Question Video Calculating the Molar Concentration of Mg(OH)₂ Using Titration Calculate Mole Ratio The coefficients of the substances in a balanced. First determine the moles of \(\ce{naoh}\) in the reaction. First determine the moles of \(\ce{naoh}\) in the reaction. From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted. Take known volume of known solution “a” and multiply it by molarity of solution a to calculate the moles of a. B using. Titration Calculate Mole Ratio.
From www.researchgate.net
Moleratio titration plots (500 MHz, [D 6 ]DMSO, 298 K) [28] showing Titration Calculate Mole Ratio B using mole ratios, determine. At ph 7, the concentration of h₃o⁺ ion and oh⁻ ion is 1:1 (titration equivalence point). A write the balanced chemical equation for the reaction and calculate the number of moles of base needed to neutralize the ascorbic acid. Next, apply the mole ratio calculation to determine the moles of. From the mole ratio, calculate. Titration Calculate Mole Ratio.
From slideplayer.com
Titrations Titration Calculations Print 1, 35, ppt download Titration Calculate Mole Ratio A write the balanced chemical equation for the reaction and calculate the number of moles of base needed to neutralize the ascorbic acid. First determine the moles of \(\ce{naoh}\) in the reaction. First determine the moles of \(\ce{naoh}\) in the reaction. 2h₂o ⇌ oh⁻ + h₃o⁺. A molar ratio is the proportion of moles of one substance to the moles. Titration Calculate Mole Ratio.
From www.youtube.com
Titration calculation example Chemistry Khan Academy YouTube Titration Calculate Mole Ratio Multiply the molarity of the strong base naoh by the volume of. From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that. B using mole ratios, determine. Next, apply the mole ratio calculation to determine the moles of. From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted. 2h₂o ⇌ oh⁻ + h₃o⁺. First determine the moles of \(\ce{naoh}\). Titration Calculate Mole Ratio.
From www.coursehero.com
[Solved] Calculate the number of moles of NaOH used in your titration Titration Calculate Mole Ratio B using mole ratios, determine. From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that. Next, apply the mole ratio calculation to determine the moles of. The coefficients of the substances in a balanced. First determine the moles of \(\ce{naoh}\) in the reaction. Multiply the molarity of the strong base naoh by the volume of. From the mole ratio, calculate. Titration Calculate Mole Ratio.
From www.slideserve.com
PPT MOLE RATIOS IN CHEMICAL EQUATIONS PowerPoint Presentation, free Titration Calculate Mole Ratio First determine the moles of \(\ce{naoh}\) in the reaction. Take known volume of known solution “a” and multiply it by molarity of solution a to calculate the moles of a. 2h₂o ⇌ oh⁻ + h₃o⁺. A write the balanced chemical equation for the reaction and calculate the number of moles of base needed to neutralize the ascorbic acid. The coefficients. Titration Calculate Mole Ratio.
From www.ck12.org
Titration (Calculations) Example 3 ( Video ) Chemistry CK12 Titration Calculate Mole Ratio First determine the moles of \(\ce{naoh}\) in the reaction. Multiply the molarity of the strong base naoh by the volume of. At ph 7, the concentration of h₃o⁺ ion and oh⁻ ion is 1:1 (titration equivalence point). The coefficients of the substances in a balanced. From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that. A titration calculation is a. Titration Calculate Mole Ratio.
From labthink.netlify.app
What is titration and how does it work Titration Calculate Mole Ratio Take known volume of known solution “a” and multiply it by molarity of solution a to calculate the moles of a. Multiply the molarity of the strong base naoh by the volume of. A molar ratio is the proportion of moles of one substance to the moles of another substance in a chemical reaction. First determine the moles of \(\ce{naoh}\). Titration Calculate Mole Ratio.
From www.youtube.com
titration calculating moles YouTube Titration Calculate Mole Ratio A molar ratio is the proportion of moles of one substance to the moles of another substance in a chemical reaction. From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted. From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that. First determine the moles of \(\ce{naoh}\) in the reaction. A write the balanced chemical equation for the reaction. Titration Calculate Mole Ratio.
From www.ck12.org
Titration (Calculations) Example 2 ( Video ) Chemistry CK12 Titration Calculate Mole Ratio 2h₂o ⇌ oh⁻ + h₃o⁺. First determine the moles of \(\ce{naoh}\) in the reaction. From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted. A titration calculation is a simple formula used to work out the concentration (in moles) of one of the reactants in a titration using the concentration of the other. A write the balanced chemical equation. Titration Calculate Mole Ratio.
From dxolxludj.blob.core.windows.net
Titration Calculating Moles at Gary Mosley blog Titration Calculate Mole Ratio Take known volume of known solution “a” and multiply it by molarity of solution a to calculate the moles of a. From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that. From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted. First determine the moles of \(\ce{naoh}\) in the reaction. The coefficients of the substances in a balanced. First. Titration Calculate Mole Ratio.
From www.youtube.com
How to Do Titration Calculations // HSC Chemistry YouTube Titration Calculate Mole Ratio The coefficients of the substances in a balanced. At ph 7, the concentration of h₃o⁺ ion and oh⁻ ion is 1:1 (titration equivalence point). A titration calculation is a simple formula used to work out the concentration (in moles) of one of the reactants in a titration using the concentration of the other. From the mole ratio, calculate the moles. Titration Calculate Mole Ratio.
From www.numerade.com
SOLVED Write a balanced equation for the corresponding titration Titration Calculate Mole Ratio 2h₂o ⇌ oh⁻ + h₃o⁺. Multiply the molarity of the strong base naoh by the volume of. First determine the moles of \(\ce{naoh}\) in the reaction. From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that. From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted. A write the balanced chemical equation for the reaction and calculate the number. Titration Calculate Mole Ratio.
From noel-kmclean.blogspot.com
Calculate Molarity of Acid in Titration Titration Calculate Mole Ratio Multiply the molarity of the strong base naoh by the volume of. First determine the moles of \(\ce{naoh}\) in the reaction. Next, apply the mole ratio calculation to determine the moles of. From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that. 2h₂o ⇌ oh⁻ + h₃o⁺. A write the balanced chemical equation for the reaction and calculate the number. Titration Calculate Mole Ratio.
From studylib.net
Molarity, moles & mass and Volumetric titration calculations Titration Calculate Mole Ratio Take known volume of known solution “a” and multiply it by molarity of solution a to calculate the moles of a. B using mole ratios, determine. Next, apply the mole ratio calculation to determine the moles of. First determine the moles of \(\ce{naoh}\) in the reaction. From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that. A titration calculation is. Titration Calculate Mole Ratio.
From www.youtube.com
Stoichiometry Problem Titration Calculation YouTube Titration Calculate Mole Ratio Take known volume of known solution “a” and multiply it by molarity of solution a to calculate the moles of a. At ph 7, the concentration of h₃o⁺ ion and oh⁻ ion is 1:1 (titration equivalence point). From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted. From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that. A titration. Titration Calculate Mole Ratio.
From slideplayer.com
Titration Calculation ppt download Titration Calculate Mole Ratio First determine the moles of \(\ce{naoh}\) in the reaction. Next, apply the mole ratio calculation to determine the moles of. At ph 7, the concentration of h₃o⁺ ion and oh⁻ ion is 1:1 (titration equivalence point). Multiply the molarity of the strong base naoh by the volume of. 2h₂o ⇌ oh⁻ + h₃o⁺. B using mole ratios, determine. A titration. Titration Calculate Mole Ratio.
From www.tes.com
Concentration and Titration Calculations GCSE Lesson (SC14c SC14d Titration Calculate Mole Ratio From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted. First determine the moles of \(\ce{naoh}\) in the reaction. The coefficients of the substances in a balanced. Multiply the molarity of the strong base naoh by the volume of. 2h₂o ⇌ oh⁻ + h₃o⁺. First determine the moles of \(\ce{naoh}\) in the reaction. B using mole ratios, determine. A. Titration Calculate Mole Ratio.
From www.chegg.com
Solved In the acidbase titration, the mole ratio between Titration Calculate Mole Ratio From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted. First determine the moles of \(\ce{naoh}\) in the reaction. From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that. At ph 7, the concentration of h₃o⁺ ion and oh⁻ ion is 1:1 (titration equivalence point). Next, apply the mole ratio calculation to determine the moles of. First determine the. Titration Calculate Mole Ratio.
From www.youtube.com
Concept of titration and titration’s Calculation lecture 5 (A level Titration Calculate Mole Ratio A titration calculation is a simple formula used to work out the concentration (in moles) of one of the reactants in a titration using the concentration of the other. A write the balanced chemical equation for the reaction and calculate the number of moles of base needed to neutralize the ascorbic acid. From the mole ratio, calculate the moles of. Titration Calculate Mole Ratio.