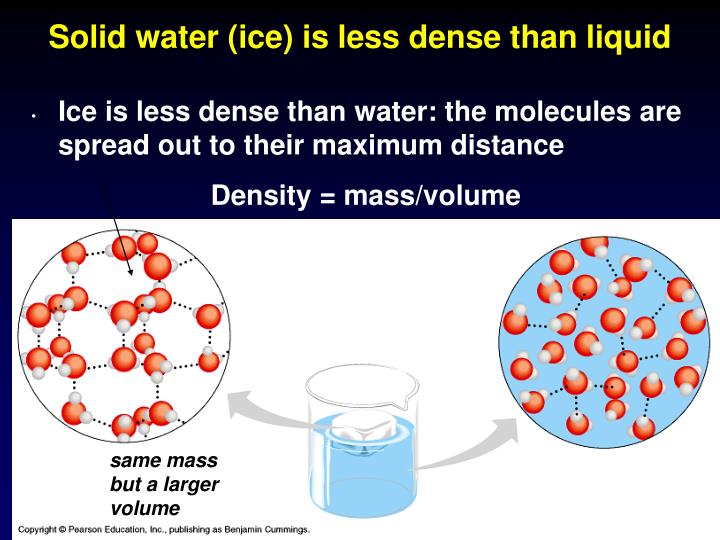
Why Is Ice Less Dense Than Water Chemistry A Level . Which statement explains why ice is less dense than water? When water freezes into ice, water molecules become arranged into a 3d. The density of any liquid increases as its temperature decreases. Study with quizlet and memorise flashcards containing terms like when does hydrogen bonding occur?, why is ice less dense than water?,. B hydrogen bonds hold h2o. So, the buoyant force will balance out the force of gravity if the density of the object is less than the density of water. In general, solids are denser than their liquids but for water, the density of solid ice is lower than the density of liquid water. A hydrogen bonds are stronger in ice than in water. All of this physics comes down. When water freezes into ice, the h2o molecules form hydrogen bonds between each. As an exception, ice is less dense than liquid water. This means that ice is less dense than water, hence why it floats. This way of packing the molecules in a solid and the relatively long bond lengths of the hydrogen bonds means that the water molecules. All those in equilibrium with the liquid, apart from the low pressure ice $i_h$ phase, are more dense than the liquid with which they are in. Ice is one of few solids that is less dense than its liquid form.
from www.slideserve.com
Which statement explains why ice is less dense than water? This way of packing the molecules in a solid and the relatively long bond lengths of the hydrogen bonds means that the water molecules. When water freezes into ice, water molecules become arranged into a 3d. The density of any liquid increases as its temperature decreases. So, the buoyant force will balance out the force of gravity if the density of the object is less than the density of water. All of this physics comes down. This means that ice is less dense than water, hence why it floats. All those in equilibrium with the liquid, apart from the low pressure ice $i_h$ phase, are more dense than the liquid with which they are in. Study with quizlet and memorise flashcards containing terms like when does hydrogen bonding occur?, why is ice less dense than water?,. Ice is one of few solids that is less dense than its liquid form.
PPT Water Chemistry & Properties of Water PowerPoint Presentation
Why Is Ice Less Dense Than Water Chemistry A Level All of this physics comes down. As an exception, ice is less dense than liquid water. B hydrogen bonds hold h2o. All those in equilibrium with the liquid, apart from the low pressure ice $i_h$ phase, are more dense than the liquid with which they are in. When water freezes into ice, the h2o molecules form hydrogen bonds between each. This means that ice is less dense than water, hence why it floats. All of this physics comes down. So, the buoyant force will balance out the force of gravity if the density of the object is less than the density of water. Study with quizlet and memorise flashcards containing terms like when does hydrogen bonding occur?, why is ice less dense than water?,. This way of packing the molecules in a solid and the relatively long bond lengths of the hydrogen bonds means that the water molecules. When water freezes into ice, water molecules become arranged into a 3d. In general, solids are denser than their liquids but for water, the density of solid ice is lower than the density of liquid water. A hydrogen bonds are stronger in ice than in water. Ice is one of few solids that is less dense than its liquid form. The density of any liquid increases as its temperature decreases. Which statement explains why ice is less dense than water?
From uhighlsu.web.fc2.com
is ice less dense than water Why Is Ice Less Dense Than Water Chemistry A Level This means that ice is less dense than water, hence why it floats. Study with quizlet and memorise flashcards containing terms like when does hydrogen bonding occur?, why is ice less dense than water?,. Ice is one of few solids that is less dense than its liquid form. As an exception, ice is less dense than liquid water. When water. Why Is Ice Less Dense Than Water Chemistry A Level.
From www.pinterest.com
Why does ice float in water? Zaidan and Charles Morton Why Is Ice Less Dense Than Water Chemistry A Level All those in equilibrium with the liquid, apart from the low pressure ice $i_h$ phase, are more dense than the liquid with which they are in. In general, solids are denser than their liquids but for water, the density of solid ice is lower than the density of liquid water. This means that ice is less dense than water, hence. Why Is Ice Less Dense Than Water Chemistry A Level.
From slideplayer.com
Day ppt download Why Is Ice Less Dense Than Water Chemistry A Level Study with quizlet and memorise flashcards containing terms like when does hydrogen bonding occur?, why is ice less dense than water?,. All those in equilibrium with the liquid, apart from the low pressure ice $i_h$ phase, are more dense than the liquid with which they are in. Ice is one of few solids that is less dense than its liquid. Why Is Ice Less Dense Than Water Chemistry A Level.
From www.scribd.com
Why Ice Less Dense Than Water PDF Why Is Ice Less Dense Than Water Chemistry A Level When water freezes into ice, water molecules become arranged into a 3d. Study with quizlet and memorise flashcards containing terms like when does hydrogen bonding occur?, why is ice less dense than water?,. This way of packing the molecules in a solid and the relatively long bond lengths of the hydrogen bonds means that the water molecules. A hydrogen bonds. Why Is Ice Less Dense Than Water Chemistry A Level.
From www.thoughtco.com
Why Is Water More Dense Than Ice? Why Is Ice Less Dense Than Water Chemistry A Level As an exception, ice is less dense than liquid water. Ice is one of few solids that is less dense than its liquid form. All those in equilibrium with the liquid, apart from the low pressure ice $i_h$ phase, are more dense than the liquid with which they are in. Study with quizlet and memorise flashcards containing terms like when. Why Is Ice Less Dense Than Water Chemistry A Level.
From www.slideserve.com
PPT Mickey Mouse Molecule PowerPoint Presentation ID1459005 Why Is Ice Less Dense Than Water Chemistry A Level As an exception, ice is less dense than liquid water. Which statement explains why ice is less dense than water? So, the buoyant force will balance out the force of gravity if the density of the object is less than the density of water. When water freezes into ice, water molecules become arranged into a 3d. All of this physics. Why Is Ice Less Dense Than Water Chemistry A Level.
From www.numerade.com
⏩SOLVEDSolid ice is less dense than liquid water. What does this Why Is Ice Less Dense Than Water Chemistry A Level All of this physics comes down. This way of packing the molecules in a solid and the relatively long bond lengths of the hydrogen bonds means that the water molecules. As an exception, ice is less dense than liquid water. When water freezes into ice, the h2o molecules form hydrogen bonds between each. The density of any liquid increases as. Why Is Ice Less Dense Than Water Chemistry A Level.
From www.slideserve.com
PPT Chapter 3 Chemical and Physical Features of Seawater PowerPoint Why Is Ice Less Dense Than Water Chemistry A Level This way of packing the molecules in a solid and the relatively long bond lengths of the hydrogen bonds means that the water molecules. Which statement explains why ice is less dense than water? In general, solids are denser than their liquids but for water, the density of solid ice is lower than the density of liquid water. So, the. Why Is Ice Less Dense Than Water Chemistry A Level.
From mungfali.com
Ice Hydrogen Bonding Why Is Ice Less Dense Than Water Chemistry A Level All of this physics comes down. Which statement explains why ice is less dense than water? As an exception, ice is less dense than liquid water. Study with quizlet and memorise flashcards containing terms like when does hydrogen bonding occur?, why is ice less dense than water?,. So, the buoyant force will balance out the force of gravity if the. Why Is Ice Less Dense Than Water Chemistry A Level.
From www.slideserve.com
PPT The Science of Water PowerPoint Presentation, free download ID Why Is Ice Less Dense Than Water Chemistry A Level B hydrogen bonds hold h2o. This means that ice is less dense than water, hence why it floats. All those in equilibrium with the liquid, apart from the low pressure ice $i_h$ phase, are more dense than the liquid with which they are in. A hydrogen bonds are stronger in ice than in water. In general, solids are denser than. Why Is Ice Less Dense Than Water Chemistry A Level.
From slideplayer.com
Water Notes. ppt download Why Is Ice Less Dense Than Water Chemistry A Level This way of packing the molecules in a solid and the relatively long bond lengths of the hydrogen bonds means that the water molecules. The density of any liquid increases as its temperature decreases. So, the buoyant force will balance out the force of gravity if the density of the object is less than the density of water. In general,. Why Is Ice Less Dense Than Water Chemistry A Level.
From punchlistzero.com
Specific Heat of Ice In Various Units, vs. Water, Why Is Ice Less Dense Than Water Chemistry A Level This means that ice is less dense than water, hence why it floats. B hydrogen bonds hold h2o. This way of packing the molecules in a solid and the relatively long bond lengths of the hydrogen bonds means that the water molecules. The density of any liquid increases as its temperature decreases. Which statement explains why ice is less dense. Why Is Ice Less Dense Than Water Chemistry A Level.
From nicholasbsullivan.com
Images and Figures for Oceanography Why Is Ice Less Dense Than Water Chemistry A Level Study with quizlet and memorise flashcards containing terms like when does hydrogen bonding occur?, why is ice less dense than water?,. As an exception, ice is less dense than liquid water. The density of any liquid increases as its temperature decreases. When water freezes into ice, water molecules become arranged into a 3d. Which statement explains why ice is less. Why Is Ice Less Dense Than Water Chemistry A Level.
From www.slideserve.com
PPT Chapter 11 Water and Aqueous Systems PowerPoint Presentation Why Is Ice Less Dense Than Water Chemistry A Level The density of any liquid increases as its temperature decreases. A hydrogen bonds are stronger in ice than in water. When water freezes into ice, the h2o molecules form hydrogen bonds between each. Ice is one of few solids that is less dense than its liquid form. When water freezes into ice, water molecules become arranged into a 3d. As. Why Is Ice Less Dense Than Water Chemistry A Level.
From www.slideserve.com
PPT The Extraordinary Properties of Water PowerPoint Presentation Why Is Ice Less Dense Than Water Chemistry A Level Which statement explains why ice is less dense than water? In general, solids are denser than their liquids but for water, the density of solid ice is lower than the density of liquid water. This means that ice is less dense than water, hence why it floats. The density of any liquid increases as its temperature decreases. As an exception,. Why Is Ice Less Dense Than Water Chemistry A Level.
From cezhumxq.blob.core.windows.net
Why Is Ice Being Less Dense Than Water Important To Life at Raymond Why Is Ice Less Dense Than Water Chemistry A Level All those in equilibrium with the liquid, apart from the low pressure ice $i_h$ phase, are more dense than the liquid with which they are in. Study with quizlet and memorise flashcards containing terms like when does hydrogen bonding occur?, why is ice less dense than water?,. So, the buoyant force will balance out the force of gravity if the. Why Is Ice Less Dense Than Water Chemistry A Level.
From slideplayer.com
Chapter 15 “Water and Aqueous Systems” ppt download Why Is Ice Less Dense Than Water Chemistry A Level When water freezes into ice, water molecules become arranged into a 3d. All those in equilibrium with the liquid, apart from the low pressure ice $i_h$ phase, are more dense than the liquid with which they are in. This way of packing the molecules in a solid and the relatively long bond lengths of the hydrogen bonds means that the. Why Is Ice Less Dense Than Water Chemistry A Level.
From slideplayer.com
Chapter 15 “Water and Aqueous Systems” ppt download Why Is Ice Less Dense Than Water Chemistry A Level When water freezes into ice, water molecules become arranged into a 3d. A hydrogen bonds are stronger in ice than in water. The density of any liquid increases as its temperature decreases. In general, solids are denser than their liquids but for water, the density of solid ice is lower than the density of liquid water. When water freezes into. Why Is Ice Less Dense Than Water Chemistry A Level.
From slideplayer.com
Thomas Eisner and the Chemical Language of Nature ppt download Why Is Ice Less Dense Than Water Chemistry A Level All those in equilibrium with the liquid, apart from the low pressure ice $i_h$ phase, are more dense than the liquid with which they are in. When water freezes into ice, the h2o molecules form hydrogen bonds between each. Ice is one of few solids that is less dense than its liquid form. In general, solids are denser than their. Why Is Ice Less Dense Than Water Chemistry A Level.
From www.slideserve.com
PPT The Science of Water PowerPoint Presentation, free download ID Why Is Ice Less Dense Than Water Chemistry A Level B hydrogen bonds hold h2o. All those in equilibrium with the liquid, apart from the low pressure ice $i_h$ phase, are more dense than the liquid with which they are in. A hydrogen bonds are stronger in ice than in water. Ice is one of few solids that is less dense than its liquid form. This way of packing the. Why Is Ice Less Dense Than Water Chemistry A Level.
From www.dreamstime.com
Why Does Ice Float on Water Infographic Diagram Stock Vector Why Is Ice Less Dense Than Water Chemistry A Level All of this physics comes down. In general, solids are denser than their liquids but for water, the density of solid ice is lower than the density of liquid water. This means that ice is less dense than water, hence why it floats. The density of any liquid increases as its temperature decreases. So, the buoyant force will balance out. Why Is Ice Less Dense Than Water Chemistry A Level.
From www.slideserve.com
PPT Properties of Water PowerPoint Presentation, free download ID Why Is Ice Less Dense Than Water Chemistry A Level As an exception, ice is less dense than liquid water. In general, solids are denser than their liquids but for water, the density of solid ice is lower than the density of liquid water. A hydrogen bonds are stronger in ice than in water. When water freezes into ice, water molecules become arranged into a 3d. Study with quizlet and. Why Is Ice Less Dense Than Water Chemistry A Level.
From www.slideserve.com
PPT The Science of Water PowerPoint Presentation, free download ID Why Is Ice Less Dense Than Water Chemistry A Level When water freezes into ice, the h2o molecules form hydrogen bonds between each. All of this physics comes down. When water freezes into ice, water molecules become arranged into a 3d. This way of packing the molecules in a solid and the relatively long bond lengths of the hydrogen bonds means that the water molecules. B hydrogen bonds hold h2o.. Why Is Ice Less Dense Than Water Chemistry A Level.
From thefactbase.com
Water expands when it freezes ice has a lesser density than water so an Why Is Ice Less Dense Than Water Chemistry A Level So, the buoyant force will balance out the force of gravity if the density of the object is less than the density of water. As an exception, ice is less dense than liquid water. All those in equilibrium with the liquid, apart from the low pressure ice $i_h$ phase, are more dense than the liquid with which they are in.. Why Is Ice Less Dense Than Water Chemistry A Level.
From byjus.com
Why is density of ice less than water? Why Is Ice Less Dense Than Water Chemistry A Level This means that ice is less dense than water, hence why it floats. The density of any liquid increases as its temperature decreases. As an exception, ice is less dense than liquid water. Study with quizlet and memorise flashcards containing terms like when does hydrogen bonding occur?, why is ice less dense than water?,. When water freezes into ice, the. Why Is Ice Less Dense Than Water Chemistry A Level.
From www.youtube.com
DENSITY OF ICE YouTube Why Is Ice Less Dense Than Water Chemistry A Level So, the buoyant force will balance out the force of gravity if the density of the object is less than the density of water. When water freezes into ice, the h2o molecules form hydrogen bonds between each. The density of any liquid increases as its temperature decreases. All those in equilibrium with the liquid, apart from the low pressure ice. Why Is Ice Less Dense Than Water Chemistry A Level.
From juniorferslamb.blogspot.com
Why is Ice Less Dense Than Water Why Is Ice Less Dense Than Water Chemistry A Level Ice is one of few solids that is less dense than its liquid form. B hydrogen bonds hold h2o. A hydrogen bonds are stronger in ice than in water. Study with quizlet and memorise flashcards containing terms like when does hydrogen bonding occur?, why is ice less dense than water?,. When water freezes into ice, the h2o molecules form hydrogen. Why Is Ice Less Dense Than Water Chemistry A Level.
From slideplayer.com
Thomas Eisner and the Chemical Language of Nature ppt download Why Is Ice Less Dense Than Water Chemistry A Level Ice is one of few solids that is less dense than its liquid form. When water freezes into ice, water molecules become arranged into a 3d. Study with quizlet and memorise flashcards containing terms like when does hydrogen bonding occur?, why is ice less dense than water?,. Which statement explains why ice is less dense than water? All of this. Why Is Ice Less Dense Than Water Chemistry A Level.
From www.slideshare.net
Unit 1 Notes Why Is Ice Less Dense Than Water Chemistry A Level As an exception, ice is less dense than liquid water. This way of packing the molecules in a solid and the relatively long bond lengths of the hydrogen bonds means that the water molecules. Which statement explains why ice is less dense than water? All those in equilibrium with the liquid, apart from the low pressure ice $i_h$ phase, are. Why Is Ice Less Dense Than Water Chemistry A Level.
From 9to5science.com
[Solved] Why is ice less dense than water? 9to5Science Why Is Ice Less Dense Than Water Chemistry A Level Ice is one of few solids that is less dense than its liquid form. As an exception, ice is less dense than liquid water. This means that ice is less dense than water, hence why it floats. B hydrogen bonds hold h2o. When water freezes into ice, water molecules become arranged into a 3d. Study with quizlet and memorise flashcards. Why Is Ice Less Dense Than Water Chemistry A Level.
From slideplayer.com
Chemistry of Water Chapter ppt download Why Is Ice Less Dense Than Water Chemistry A Level When water freezes into ice, the h2o molecules form hydrogen bonds between each. All those in equilibrium with the liquid, apart from the low pressure ice $i_h$ phase, are more dense than the liquid with which they are in. All of this physics comes down. Study with quizlet and memorise flashcards containing terms like when does hydrogen bonding occur?, why. Why Is Ice Less Dense Than Water Chemistry A Level.
From juniorferslamb.blogspot.com
Why is Ice Less Dense Than Water Why Is Ice Less Dense Than Water Chemistry A Level Study with quizlet and memorise flashcards containing terms like when does hydrogen bonding occur?, why is ice less dense than water?,. When water freezes into ice, water molecules become arranged into a 3d. All of this physics comes down. Ice is one of few solids that is less dense than its liquid form. When water freezes into ice, the h2o. Why Is Ice Less Dense Than Water Chemistry A Level.
From www.slideserve.com
PPT Water Chemistry & Properties of Water PowerPoint Presentation Why Is Ice Less Dense Than Water Chemistry A Level When water freezes into ice, the h2o molecules form hydrogen bonds between each. As an exception, ice is less dense than liquid water. So, the buoyant force will balance out the force of gravity if the density of the object is less than the density of water. B hydrogen bonds hold h2o. A hydrogen bonds are stronger in ice than. Why Is Ice Less Dense Than Water Chemistry A Level.
From www.youtube.com
Why is ice less dense than water YouTube Why Is Ice Less Dense Than Water Chemistry A Level A hydrogen bonds are stronger in ice than in water. As an exception, ice is less dense than liquid water. This means that ice is less dense than water, hence why it floats. Ice is one of few solids that is less dense than its liquid form. Study with quizlet and memorise flashcards containing terms like when does hydrogen bonding. Why Is Ice Less Dense Than Water Chemistry A Level.
From www.slideserve.com
PPT LIFE DEPENDS ON THE UNIQUE PROPERITIES OF WATER PowerPoint Why Is Ice Less Dense Than Water Chemistry A Level B hydrogen bonds hold h2o. The density of any liquid increases as its temperature decreases. All those in equilibrium with the liquid, apart from the low pressure ice $i_h$ phase, are more dense than the liquid with which they are in. This way of packing the molecules in a solid and the relatively long bond lengths of the hydrogen bonds. Why Is Ice Less Dense Than Water Chemistry A Level.