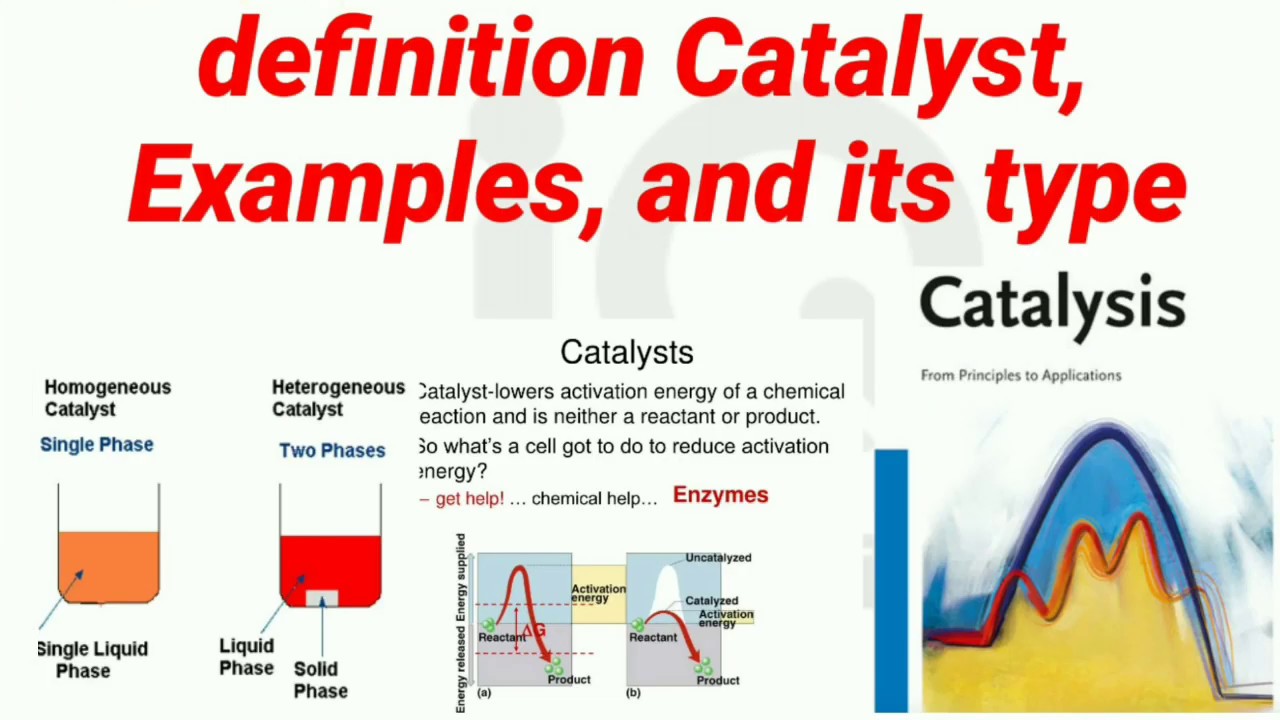
Catalyst Ap Bio Definition . • catalyst, catalysis, and catalase. A substance that helps a chemical reaction to occur is a catalyst, and the special molecules that catalyze biochemical reactions are called. After doing this lab you should be able to: Enzymes are proteins produced by living cells. What is the difference between kinetic and potential energy? In this section, you will explore the following questions: A catalyst is a substance that increases the rate of a chemical reaction without being consumed in the. A catalyst is a substance that increases the rate of a chemical reaction without being consumed or altered in the process. They are biochemical catalysts meaning they lower the activation energy needed for a biochemical. A catalyst is any substance that speeds up the rate of a reaction, most commonly by providing the necessary activation energy for a reaction to take place. • measure the effects of changes in temperature, ph, enzyme.
from medicaltaste.weebly.com
A catalyst is any substance that speeds up the rate of a reaction, most commonly by providing the necessary activation energy for a reaction to take place. A catalyst is a substance that increases the rate of a chemical reaction without being consumed in the. After doing this lab you should be able to: • measure the effects of changes in temperature, ph, enzyme. Enzymes are proteins produced by living cells. A substance that helps a chemical reaction to occur is a catalyst, and the special molecules that catalyze biochemical reactions are called. • catalyst, catalysis, and catalase. In this section, you will explore the following questions: What is the difference between kinetic and potential energy? They are biochemical catalysts meaning they lower the activation energy needed for a biochemical.
Periodic table catalyst definition chemistry medicaltaste
Catalyst Ap Bio Definition A substance that helps a chemical reaction to occur is a catalyst, and the special molecules that catalyze biochemical reactions are called. • measure the effects of changes in temperature, ph, enzyme. A substance that helps a chemical reaction to occur is a catalyst, and the special molecules that catalyze biochemical reactions are called. A catalyst is a substance that increases the rate of a chemical reaction without being consumed or altered in the process. In this section, you will explore the following questions: What is the difference between kinetic and potential energy? They are biochemical catalysts meaning they lower the activation energy needed for a biochemical. A catalyst is a substance that increases the rate of a chemical reaction without being consumed in the. After doing this lab you should be able to: Enzymes are proteins produced by living cells. A catalyst is any substance that speeds up the rate of a reaction, most commonly by providing the necessary activation energy for a reaction to take place. • catalyst, catalysis, and catalase.
From www.slideserve.com
PPT Reaction Rate PowerPoint Presentation, free download ID3534068 Catalyst Ap Bio Definition Enzymes are proteins produced by living cells. • catalyst, catalysis, and catalase. In this section, you will explore the following questions: A catalyst is a substance that increases the rate of a chemical reaction without being consumed in the. A catalyst is a substance that increases the rate of a chemical reaction without being consumed or altered in the process.. Catalyst Ap Bio Definition.
From www.youtube.com
Enzyme Catalysis How Do Enzymes Work? AP Biology 3.2 YouTube Catalyst Ap Bio Definition After doing this lab you should be able to: A catalyst is any substance that speeds up the rate of a reaction, most commonly by providing the necessary activation energy for a reaction to take place. In this section, you will explore the following questions: • catalyst, catalysis, and catalase. Enzymes are proteins produced by living cells. • measure the. Catalyst Ap Bio Definition.
From www.pinterest.com
Homogeneous Catalyst Easy Science Ap chemistry, Chemical equation, Chemical reactions Catalyst Ap Bio Definition Enzymes are proteins produced by living cells. • catalyst, catalysis, and catalase. After doing this lab you should be able to: In this section, you will explore the following questions: A catalyst is a substance that increases the rate of a chemical reaction without being consumed in the. A catalyst is any substance that speeds up the rate of a. Catalyst Ap Bio Definition.
From studylib.net
AP Biology Lab 2 Enzyme Catalysis Catalyst Ap Bio Definition In this section, you will explore the following questions: A catalyst is a substance that increases the rate of a chemical reaction without being consumed or altered in the process. A catalyst is any substance that speeds up the rate of a reaction, most commonly by providing the necessary activation energy for a reaction to take place. A substance that. Catalyst Ap Bio Definition.
From www.slideserve.com
PPT AP Biology PowerPoint Presentation ID302778 Catalyst Ap Bio Definition • catalyst, catalysis, and catalase. A catalyst is a substance that increases the rate of a chemical reaction without being consumed or altered in the process. Enzymes are proteins produced by living cells. After doing this lab you should be able to: • measure the effects of changes in temperature, ph, enzyme. In this section, you will explore the following. Catalyst Ap Bio Definition.
From www.thoughtco.com
Catalysis Definition in Chemistry Catalyst Ap Bio Definition • measure the effects of changes in temperature, ph, enzyme. In this section, you will explore the following questions: A catalyst is any substance that speeds up the rate of a reaction, most commonly by providing the necessary activation energy for a reaction to take place. • catalyst, catalysis, and catalase. A catalyst is a substance that increases the rate. Catalyst Ap Bio Definition.
From www.slideserve.com
PPT AP Biology PowerPoint Presentation ID302778 Catalyst Ap Bio Definition In this section, you will explore the following questions: What is the difference between kinetic and potential energy? A catalyst is any substance that speeds up the rate of a reaction, most commonly by providing the necessary activation energy for a reaction to take place. • catalyst, catalysis, and catalase. A substance that helps a chemical reaction to occur is. Catalyst Ap Bio Definition.
From www.slideserve.com
PPT AP Biology PowerPoint Presentation, free download ID4859041 Catalyst Ap Bio Definition What is the difference between kinetic and potential energy? A catalyst is any substance that speeds up the rate of a reaction, most commonly by providing the necessary activation energy for a reaction to take place. In this section, you will explore the following questions: After doing this lab you should be able to: A catalyst is a substance that. Catalyst Ap Bio Definition.
From studylib.net
AP Biology Enzyme Catalysis Inquiry Lab Backgro Catalyst Ap Bio Definition After doing this lab you should be able to: • measure the effects of changes in temperature, ph, enzyme. A catalyst is a substance that increases the rate of a chemical reaction without being consumed in the. In this section, you will explore the following questions: Enzymes are proteins produced by living cells. A catalyst is a substance that increases. Catalyst Ap Bio Definition.
From exozpzccv.blob.core.windows.net
Catalyst Biology Simple Definition at Lydia Hatcher blog Catalyst Ap Bio Definition They are biochemical catalysts meaning they lower the activation energy needed for a biochemical. • catalyst, catalysis, and catalase. A catalyst is a substance that increases the rate of a chemical reaction without being consumed in the. Enzymes are proteins produced by living cells. A catalyst is a substance that increases the rate of a chemical reaction without being consumed. Catalyst Ap Bio Definition.
From www.slideserve.com
PPT Role of Catalysts PowerPoint Presentation, free download ID2823169 Catalyst Ap Bio Definition A catalyst is a substance that increases the rate of a chemical reaction without being consumed in the. A substance that helps a chemical reaction to occur is a catalyst, and the special molecules that catalyze biochemical reactions are called. Enzymes are proteins produced by living cells. A catalyst is a substance that increases the rate of a chemical reaction. Catalyst Ap Bio Definition.
From www.slideserve.com
PPT Enzymes as Biological Catalysts PowerPoint Presentation, free download ID2683753 Catalyst Ap Bio Definition • measure the effects of changes in temperature, ph, enzyme. A catalyst is any substance that speeds up the rate of a reaction, most commonly by providing the necessary activation energy for a reaction to take place. A catalyst is a substance that increases the rate of a chemical reaction without being consumed in the. After doing this lab you. Catalyst Ap Bio Definition.
From www.slideserve.com
PPT Enzyme Review PowerPoint Presentation, free download ID4314407 Catalyst Ap Bio Definition • measure the effects of changes in temperature, ph, enzyme. A catalyst is a substance that increases the rate of a chemical reaction without being consumed in the. After doing this lab you should be able to: A catalyst is a substance that increases the rate of a chemical reaction without being consumed or altered in the process. They are. Catalyst Ap Bio Definition.
From medicaltaste.weebly.com
Periodic table catalyst definition chemistry medicaltaste Catalyst Ap Bio Definition After doing this lab you should be able to: In this section, you will explore the following questions: A catalyst is any substance that speeds up the rate of a reaction, most commonly by providing the necessary activation energy for a reaction to take place. A catalyst is a substance that increases the rate of a chemical reaction without being. Catalyst Ap Bio Definition.
From slideplayer.com
Biological catalysts Enzymes IGCSE Biology. ppt download Catalyst Ap Bio Definition A catalyst is a substance that increases the rate of a chemical reaction without being consumed or altered in the process. A substance that helps a chemical reaction to occur is a catalyst, and the special molecules that catalyze biochemical reactions are called. What is the difference between kinetic and potential energy? A catalyst is any substance that speeds up. Catalyst Ap Bio Definition.
From www.slideserve.com
PPT AP Biology PowerPoint Presentation ID302778 Catalyst Ap Bio Definition A substance that helps a chemical reaction to occur is a catalyst, and the special molecules that catalyze biochemical reactions are called. • measure the effects of changes in temperature, ph, enzyme. A catalyst is a substance that increases the rate of a chemical reaction without being consumed in the. After doing this lab you should be able to: In. Catalyst Ap Bio Definition.
From www.scribd.com
Biological Catalysts (13) Hydrolysis Enzyme Catalyst Ap Bio Definition Enzymes are proteins produced by living cells. After doing this lab you should be able to: • catalyst, catalysis, and catalase. In this section, you will explore the following questions: • measure the effects of changes in temperature, ph, enzyme. What is the difference between kinetic and potential energy? They are biochemical catalysts meaning they lower the activation energy needed. Catalyst Ap Bio Definition.
From www.slideserve.com
PPT Chapter 2Enzymes PowerPoint Presentation, free download ID5566824 Catalyst Ap Bio Definition Enzymes are proteins produced by living cells. After doing this lab you should be able to: What is the difference between kinetic and potential energy? A catalyst is a substance that increases the rate of a chemical reaction without being consumed in the. A catalyst is a substance that increases the rate of a chemical reaction without being consumed or. Catalyst Ap Bio Definition.
From elizabethsapbioblog.blogspot.com
AP BIOLOGY Enzyme Catalysis Lab Catalyst Ap Bio Definition • catalyst, catalysis, and catalase. Enzymes are proteins produced by living cells. A catalyst is a substance that increases the rate of a chemical reaction without being consumed or altered in the process. A catalyst is a substance that increases the rate of a chemical reaction without being consumed in the. They are biochemical catalysts meaning they lower the activation. Catalyst Ap Bio Definition.
From www.youtube.com
Definition of Catalyst Chemical Chemistry Class 12 YouTube Catalyst Ap Bio Definition In this section, you will explore the following questions: What is the difference between kinetic and potential energy? A catalyst is a substance that increases the rate of a chemical reaction without being consumed in the. A catalyst is any substance that speeds up the rate of a reaction, most commonly by providing the necessary activation energy for a reaction. Catalyst Ap Bio Definition.
From www.studocu.com
Ap bio enzyme Catalase Lab AP AP BIO ENZYME ACTIVITY This activity is an alternative to the Catalyst Ap Bio Definition A catalyst is a substance that increases the rate of a chemical reaction without being consumed or altered in the process. A catalyst is any substance that speeds up the rate of a reaction, most commonly by providing the necessary activation energy for a reaction to take place. • measure the effects of changes in temperature, ph, enzyme. Enzymes are. Catalyst Ap Bio Definition.
From www.scribd.com
AP BIO Enzyme Catalysis Lab Report PDF Hydrogen Peroxide Chemical Reactions Catalyst Ap Bio Definition What is the difference between kinetic and potential energy? A substance that helps a chemical reaction to occur is a catalyst, and the special molecules that catalyze biochemical reactions are called. • measure the effects of changes in temperature, ph, enzyme. After doing this lab you should be able to: Enzymes are proteins produced by living cells. A catalyst is. Catalyst Ap Bio Definition.
From www.youtube.com
AP Biology Lab 2 Enzyme Catalysis YouTube Catalyst Ap Bio Definition After doing this lab you should be able to: • catalyst, catalysis, and catalase. Enzymes are proteins produced by living cells. What is the difference between kinetic and potential energy? • measure the effects of changes in temperature, ph, enzyme. A catalyst is a substance that increases the rate of a chemical reaction without being consumed or altered in the. Catalyst Ap Bio Definition.
From www.researchgate.net
1 Schematic illustration of a catalytic process showing "A" and "B"... Download Scientific Diagram Catalyst Ap Bio Definition Enzymes are proteins produced by living cells. After doing this lab you should be able to: What is the difference between kinetic and potential energy? • measure the effects of changes in temperature, ph, enzyme. • catalyst, catalysis, and catalase. In this section, you will explore the following questions: They are biochemical catalysts meaning they lower the activation energy needed. Catalyst Ap Bio Definition.
From www.slideserve.com
PPT Catalyst PowerPoint Presentation, free download ID257707 Catalyst Ap Bio Definition • catalyst, catalysis, and catalase. A catalyst is a substance that increases the rate of a chemical reaction without being consumed or altered in the process. A substance that helps a chemical reaction to occur is a catalyst, and the special molecules that catalyze biochemical reactions are called. A catalyst is any substance that speeds up the rate of a. Catalyst Ap Bio Definition.
From www.slideserve.com
PPT CATALYSIS AND CATALYTIC REACTION MECHANISM PART 1 PowerPoint Presentation ID1988156 Catalyst Ap Bio Definition What is the difference between kinetic and potential energy? A catalyst is a substance that increases the rate of a chemical reaction without being consumed in the. They are biochemical catalysts meaning they lower the activation energy needed for a biochemical. A catalyst is a substance that increases the rate of a chemical reaction without being consumed or altered in. Catalyst Ap Bio Definition.
From www.slideserve.com
PPT 23.5 Features of homogeneous catalysis PowerPoint Presentation ID377097 Catalyst Ap Bio Definition A catalyst is any substance that speeds up the rate of a reaction, most commonly by providing the necessary activation energy for a reaction to take place. • measure the effects of changes in temperature, ph, enzyme. After doing this lab you should be able to: A catalyst is a substance that increases the rate of a chemical reaction without. Catalyst Ap Bio Definition.
From www.slideshare.net
Biology 2.4 Catalyst Ap Bio Definition A catalyst is a substance that increases the rate of a chemical reaction without being consumed or altered in the process. A catalyst is any substance that speeds up the rate of a reaction, most commonly by providing the necessary activation energy for a reaction to take place. Enzymes are proteins produced by living cells. A substance that helps a. Catalyst Ap Bio Definition.
From www.slideserve.com
PPT Enzymes Biological Catalysts PowerPoint Presentation, free download ID5736986 Catalyst Ap Bio Definition Enzymes are proteins produced by living cells. A catalyst is any substance that speeds up the rate of a reaction, most commonly by providing the necessary activation energy for a reaction to take place. They are biochemical catalysts meaning they lower the activation energy needed for a biochemical. In this section, you will explore the following questions: What is the. Catalyst Ap Bio Definition.
From www.slideserve.com
PPT Catalyst PowerPoint Presentation, free download ID257707 Catalyst Ap Bio Definition A catalyst is a substance that increases the rate of a chemical reaction without being consumed in the. They are biochemical catalysts meaning they lower the activation energy needed for a biochemical. A substance that helps a chemical reaction to occur is a catalyst, and the special molecules that catalyze biochemical reactions are called. A catalyst is any substance that. Catalyst Ap Bio Definition.
From sciencenotes.org
What Is a Catalyst? Understand Catalysis Catalyst Ap Bio Definition In this section, you will explore the following questions: What is the difference between kinetic and potential energy? • catalyst, catalysis, and catalase. After doing this lab you should be able to: A catalyst is a substance that increases the rate of a chemical reaction without being consumed or altered in the process. • measure the effects of changes in. Catalyst Ap Bio Definition.
From www.expii.com
Catalysts (Enzymes) — Overview & Examples Expii Catalyst Ap Bio Definition • measure the effects of changes in temperature, ph, enzyme. A substance that helps a chemical reaction to occur is a catalyst, and the special molecules that catalyze biochemical reactions are called. Enzymes are proteins produced by living cells. • catalyst, catalysis, and catalase. A catalyst is any substance that speeds up the rate of a reaction, most commonly by. Catalyst Ap Bio Definition.
From www.flinnsci.com
Enzyme Catalysis—Classic Lab Kit for AP® Biology, 8 Groups Flinn Scientific Catalyst Ap Bio Definition They are biochemical catalysts meaning they lower the activation energy needed for a biochemical. A catalyst is any substance that speeds up the rate of a reaction, most commonly by providing the necessary activation energy for a reaction to take place. Enzymes are proteins produced by living cells. A catalyst is a substance that increases the rate of a chemical. Catalyst Ap Bio Definition.
From www.youtube.com
AP Bio Enzyme Catalysis Demonstration YouTube Catalyst Ap Bio Definition A substance that helps a chemical reaction to occur is a catalyst, and the special molecules that catalyze biochemical reactions are called. A catalyst is a substance that increases the rate of a chemical reaction without being consumed in the. After doing this lab you should be able to: In this section, you will explore the following questions: Enzymes are. Catalyst Ap Bio Definition.
From www.worksheetsplanet.com
What is a Catalyst Definition of Catalyst Catalyst Ap Bio Definition After doing this lab you should be able to: In this section, you will explore the following questions: • catalyst, catalysis, and catalase. Enzymes are proteins produced by living cells. A catalyst is a substance that increases the rate of a chemical reaction without being consumed or altered in the process. A substance that helps a chemical reaction to occur. Catalyst Ap Bio Definition.