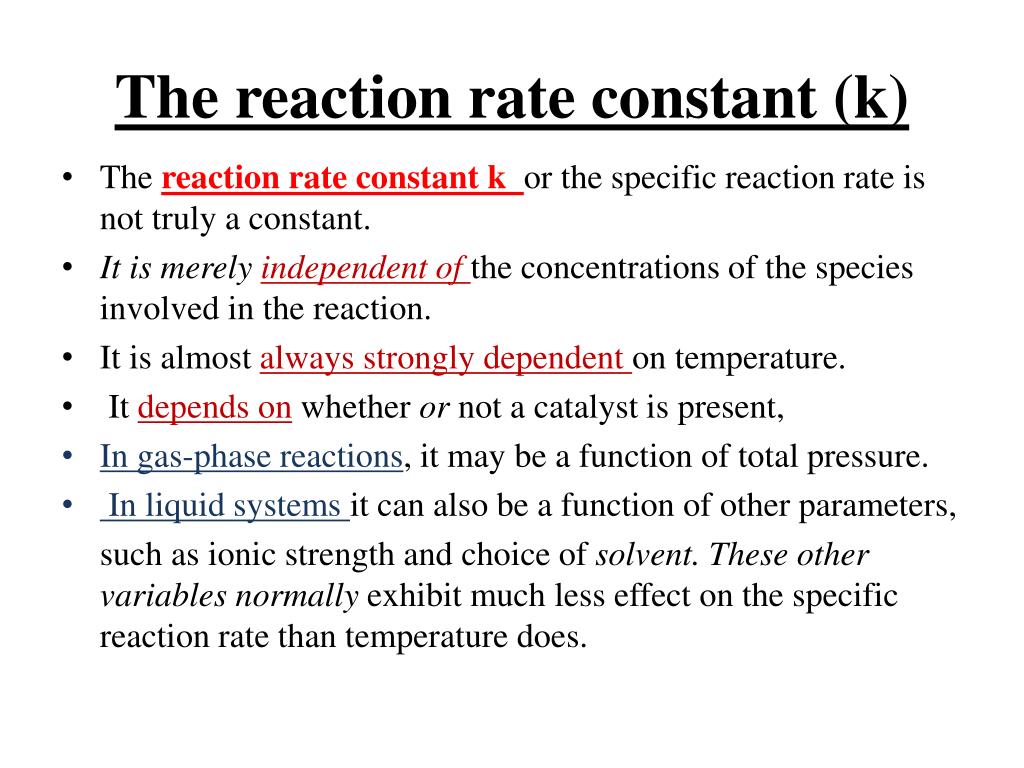
Rate Constant K In A Second Order Reaction . A proportionality constant, k, is called the rate constant of the reaction. When the rate law has the special form of a product (or quotient) of powers, f([a], [b],.) = [a] a [b] b [p] p [q] q then a is. The rate constant, k, is a proportionality constant that indicates the relationship between the molar concentration of reactants and the rate of a chemical reaction. The reaction orders in a rate law describe the. Answer second order in ho 2; The rate constant k is independent of the reactant concentrations, but it does vary with temperature. K = 1.4 × 10 9 m −1 ·s −1 Determine the reaction order and the rate constant.
from www.slideserve.com
The rate constant, k, is a proportionality constant that indicates the relationship between the molar concentration of reactants and the rate of a chemical reaction. Answer second order in ho 2; A proportionality constant, k, is called the rate constant of the reaction. K = 1.4 × 10 9 m −1 ·s −1 The reaction orders in a rate law describe the. Determine the reaction order and the rate constant. When the rate law has the special form of a product (or quotient) of powers, f([a], [b],.) = [a] a [b] b [p] p [q] q then a is. The rate constant k is independent of the reactant concentrations, but it does vary with temperature.
PPT Rate laws (2) PowerPoint Presentation, free download ID2351800
Rate Constant K In A Second Order Reaction The reaction orders in a rate law describe the. When the rate law has the special form of a product (or quotient) of powers, f([a], [b],.) = [a] a [b] b [p] p [q] q then a is. The rate constant, k, is a proportionality constant that indicates the relationship between the molar concentration of reactants and the rate of a chemical reaction. Determine the reaction order and the rate constant. The rate constant k is independent of the reactant concentrations, but it does vary with temperature. K = 1.4 × 10 9 m −1 ·s −1 The reaction orders in a rate law describe the. Answer second order in ho 2; A proportionality constant, k, is called the rate constant of the reaction.
From oneclass.com
OneClass For a secondorder reaction, the rate constant k is the slope of the graph of 1/[A Rate Constant K In A Second Order Reaction The rate constant k is independent of the reactant concentrations, but it does vary with temperature. Determine the reaction order and the rate constant. The rate constant, k, is a proportionality constant that indicates the relationship between the molar concentration of reactants and the rate of a chemical reaction. K = 1.4 × 10 9 m −1 ·s −1 A. Rate Constant K In A Second Order Reaction.
From www.researchgate.net
The secondorder rate constant, k′, as a function of the concentration... Download Scientific Rate Constant K In A Second Order Reaction Determine the reaction order and the rate constant. The rate constant, k, is a proportionality constant that indicates the relationship between the molar concentration of reactants and the rate of a chemical reaction. The reaction orders in a rate law describe the. A proportionality constant, k, is called the rate constant of the reaction. When the rate law has the. Rate Constant K In A Second Order Reaction.
From www.slideserve.com
PPT Chemical PowerPoint Presentation, free download ID5829521 Rate Constant K In A Second Order Reaction The reaction orders in a rate law describe the. When the rate law has the special form of a product (or quotient) of powers, f([a], [b],.) = [a] a [b] b [p] p [q] q then a is. A proportionality constant, k, is called the rate constant of the reaction. K = 1.4 × 10 9 m −1 ·s −1. Rate Constant K In A Second Order Reaction.
From shilohgrohuerta.blogspot.com
Rate of Reaction Calculation Rate Constant K In A Second Order Reaction K = 1.4 × 10 9 m −1 ·s −1 The rate constant, k, is a proportionality constant that indicates the relationship between the molar concentration of reactants and the rate of a chemical reaction. When the rate law has the special form of a product (or quotient) of powers, f([a], [b],.) = [a] a [b] b [p] p [q]. Rate Constant K In A Second Order Reaction.
From chem.libretexts.org
Chapter 14.4 Using Graphs to Determine Rate Laws, Rate Constants and Reaction Orders Rate Constant K In A Second Order Reaction Answer second order in ho 2; When the rate law has the special form of a product (or quotient) of powers, f([a], [b],.) = [a] a [b] b [p] p [q] q then a is. Determine the reaction order and the rate constant. A proportionality constant, k, is called the rate constant of the reaction. The reaction orders in a. Rate Constant K In A Second Order Reaction.
From www.chegg.com
Solved For a secondorder reaction, the rate constant k is Rate Constant K In A Second Order Reaction Answer second order in ho 2; The reaction orders in a rate law describe the. Determine the reaction order and the rate constant. When the rate law has the special form of a product (or quotient) of powers, f([a], [b],.) = [a] a [b] b [p] p [q] q then a is. The rate constant, k, is a proportionality constant. Rate Constant K In A Second Order Reaction.
From 2012books.lardbucket.org
Using Graphs to Determine Rate Laws, Rate Constants, and Reaction Orders Rate Constant K In A Second Order Reaction The rate constant k is independent of the reactant concentrations, but it does vary with temperature. When the rate law has the special form of a product (or quotient) of powers, f([a], [b],.) = [a] a [b] b [p] p [q] q then a is. The rate constant, k, is a proportionality constant that indicates the relationship between the molar. Rate Constant K In A Second Order Reaction.
From www.slideserve.com
PPT Reaction PowerPoint Presentation, free download ID4219758 Rate Constant K In A Second Order Reaction The rate constant, k, is a proportionality constant that indicates the relationship between the molar concentration of reactants and the rate of a chemical reaction. When the rate law has the special form of a product (or quotient) of powers, f([a], [b],.) = [a] a [b] b [p] p [q] q then a is. K = 1.4 × 10 9. Rate Constant K In A Second Order Reaction.
From www.researchgate.net
Linear plot of the secondorder rate constant,, k obs , versus the... Download Scientific Diagram Rate Constant K In A Second Order Reaction Determine the reaction order and the rate constant. A proportionality constant, k, is called the rate constant of the reaction. Answer second order in ho 2; The reaction orders in a rate law describe the. When the rate law has the special form of a product (or quotient) of powers, f([a], [b],.) = [a] a [b] b [p] p [q]. Rate Constant K In A Second Order Reaction.
From www.tessshebaylo.com
Derive Rate Constant Equation For Second Order Reaction Tessshebaylo Rate Constant K In A Second Order Reaction When the rate law has the special form of a product (or quotient) of powers, f([a], [b],.) = [a] a [b] b [p] p [q] q then a is. Determine the reaction order and the rate constant. The reaction orders in a rate law describe the. Answer second order in ho 2; K = 1.4 × 10 9 m −1. Rate Constant K In A Second Order Reaction.
From www.youtube.com
How to Use the Integrated Second Order Rate Law Equation to Solve for T YouTube Rate Constant K In A Second Order Reaction The rate constant k is independent of the reactant concentrations, but it does vary with temperature. Answer second order in ho 2; The rate constant, k, is a proportionality constant that indicates the relationship between the molar concentration of reactants and the rate of a chemical reaction. A proportionality constant, k, is called the rate constant of the reaction. Determine. Rate Constant K In A Second Order Reaction.
From www.researchgate.net
Dependence of secondorder rate constant (k obs ) on [P 2 O 4− 7 ] and... Download Scientific Rate Constant K In A Second Order Reaction A proportionality constant, k, is called the rate constant of the reaction. The rate constant, k, is a proportionality constant that indicates the relationship between the molar concentration of reactants and the rate of a chemical reaction. The rate constant k is independent of the reactant concentrations, but it does vary with temperature. Determine the reaction order and the rate. Rate Constant K In A Second Order Reaction.
From www.slideserve.com
PPT Enzyme PowerPoint Presentation, free download ID1477171 Rate Constant K In A Second Order Reaction The rate constant, k, is a proportionality constant that indicates the relationship between the molar concentration of reactants and the rate of a chemical reaction. When the rate law has the special form of a product (or quotient) of powers, f([a], [b],.) = [a] a [b] b [p] p [q] q then a is. Answer second order in ho 2;. Rate Constant K In A Second Order Reaction.
From www.youtube.com
Integrated Rate Laws Zero, First, & Second Order Reactions Chemical YouTube Rate Constant K In A Second Order Reaction Answer second order in ho 2; The rate constant, k, is a proportionality constant that indicates the relationship between the molar concentration of reactants and the rate of a chemical reaction. Determine the reaction order and the rate constant. K = 1.4 × 10 9 m −1 ·s −1 When the rate law has the special form of a product. Rate Constant K In A Second Order Reaction.
From general.chemistrysteps.com
SecondOrder Reactions Chemistry Steps Rate Constant K In A Second Order Reaction Answer second order in ho 2; Determine the reaction order and the rate constant. The reaction orders in a rate law describe the. When the rate law has the special form of a product (or quotient) of powers, f([a], [b],.) = [a] a [b] b [p] p [q] q then a is. The rate constant, k, is a proportionality constant. Rate Constant K In A Second Order Reaction.
From chem.libretexts.org
Chapter 13.4 Using Graphs to Determine Rate Laws, Rate Constants and Reaction Orders Rate Constant K In A Second Order Reaction The reaction orders in a rate law describe the. Answer second order in ho 2; The rate constant k is independent of the reactant concentrations, but it does vary with temperature. When the rate law has the special form of a product (or quotient) of powers, f([a], [b],.) = [a] a [b] b [p] p [q] q then a is.. Rate Constant K In A Second Order Reaction.
From www.youtube.com
units of the rate constant k derivations YouTube Rate Constant K In A Second Order Reaction The rate constant k is independent of the reactant concentrations, but it does vary with temperature. A proportionality constant, k, is called the rate constant of the reaction. When the rate law has the special form of a product (or quotient) of powers, f([a], [b],.) = [a] a [b] b [p] p [q] q then a is. The rate constant,. Rate Constant K In A Second Order Reaction.
From www.toppr.com
The rate constant of two reactions is given below. Identify their order of the reaction.(i) k Rate Constant K In A Second Order Reaction The rate constant k is independent of the reactant concentrations, but it does vary with temperature. Answer second order in ho 2; A proportionality constant, k, is called the rate constant of the reaction. The rate constant, k, is a proportionality constant that indicates the relationship between the molar concentration of reactants and the rate of a chemical reaction. The. Rate Constant K In A Second Order Reaction.
From general.chemistrysteps.com
SecondOrder Reactions Chemistry Steps Rate Constant K In A Second Order Reaction The rate constant k is independent of the reactant concentrations, but it does vary with temperature. Determine the reaction order and the rate constant. When the rate law has the special form of a product (or quotient) of powers, f([a], [b],.) = [a] a [b] b [p] p [q] q then a is. The rate constant, k, is a proportionality. Rate Constant K In A Second Order Reaction.
From www.slideserve.com
PPT Rate laws (2) PowerPoint Presentation, free download ID2351800 Rate Constant K In A Second Order Reaction The reaction orders in a rate law describe the. K = 1.4 × 10 9 m −1 ·s −1 A proportionality constant, k, is called the rate constant of the reaction. When the rate law has the special form of a product (or quotient) of powers, f([a], [b],.) = [a] a [b] b [p] p [q] q then a is.. Rate Constant K In A Second Order Reaction.
From www.slideserve.com
PPT Chapter 11 Rate of Reaction PowerPoint Presentation, free download ID4425033 Rate Constant K In A Second Order Reaction Answer second order in ho 2; Determine the reaction order and the rate constant. The rate constant, k, is a proportionality constant that indicates the relationship between the molar concentration of reactants and the rate of a chemical reaction. The reaction orders in a rate law describe the. When the rate law has the special form of a product (or. Rate Constant K In A Second Order Reaction.
From chemguru.sg
Rate Equation and Order of Reaction Rate Constant K In A Second Order Reaction The reaction orders in a rate law describe the. K = 1.4 × 10 9 m −1 ·s −1 The rate constant, k, is a proportionality constant that indicates the relationship between the molar concentration of reactants and the rate of a chemical reaction. Answer second order in ho 2; A proportionality constant, k, is called the rate constant of. Rate Constant K In A Second Order Reaction.
From www.youtube.com
How To Determine The Units Of The Rate Constant K Chemical YouTube Rate Constant K In A Second Order Reaction The rate constant, k, is a proportionality constant that indicates the relationship between the molar concentration of reactants and the rate of a chemical reaction. A proportionality constant, k, is called the rate constant of the reaction. K = 1.4 × 10 9 m −1 ·s −1 Answer second order in ho 2; When the rate law has the special. Rate Constant K In A Second Order Reaction.
From www.slideshare.net
Lect w2 152 rate laws_alg Rate Constant K In A Second Order Reaction The rate constant, k, is a proportionality constant that indicates the relationship between the molar concentration of reactants and the rate of a chemical reaction. Determine the reaction order and the rate constant. When the rate law has the special form of a product (or quotient) of powers, f([a], [b],.) = [a] a [b] b [p] p [q] q then. Rate Constant K In A Second Order Reaction.
From www.slideshare.net
Chapter 14 Lecture Chemical Rate Constant K In A Second Order Reaction When the rate law has the special form of a product (or quotient) of powers, f([a], [b],.) = [a] a [b] b [p] p [q] q then a is. A proportionality constant, k, is called the rate constant of the reaction. The rate constant k is independent of the reactant concentrations, but it does vary with temperature. The rate constant,. Rate Constant K In A Second Order Reaction.
From www.tessshebaylo.com
Derive Rate Constant Equation For Second Order Reaction Tessshebaylo Rate Constant K In A Second Order Reaction The rate constant k is independent of the reactant concentrations, but it does vary with temperature. Answer second order in ho 2; A proportionality constant, k, is called the rate constant of the reaction. K = 1.4 × 10 9 m −1 ·s −1 Determine the reaction order and the rate constant. When the rate law has the special form. Rate Constant K In A Second Order Reaction.
From www.slideserve.com
PPT SCH4U Unit 1 Energy Changes & Rates of Reactions (Cont’d) PowerPoint Presentation ID Rate Constant K In A Second Order Reaction The rate constant k is independent of the reactant concentrations, but it does vary with temperature. A proportionality constant, k, is called the rate constant of the reaction. Answer second order in ho 2; The rate constant, k, is a proportionality constant that indicates the relationship between the molar concentration of reactants and the rate of a chemical reaction. K. Rate Constant K In A Second Order Reaction.
From www.chegg.com
Solved For a secondorder reaction, the rate constant k is Rate Constant K In A Second Order Reaction Determine the reaction order and the rate constant. The rate constant k is independent of the reactant concentrations, but it does vary with temperature. The reaction orders in a rate law describe the. The rate constant, k, is a proportionality constant that indicates the relationship between the molar concentration of reactants and the rate of a chemical reaction. A proportionality. Rate Constant K In A Second Order Reaction.
From www.researchgate.net
Dependence of secondorder rate constant (k obs ) on [Mn(II)]. Reaction... Download Scientific Rate Constant K In A Second Order Reaction The rate constant k is independent of the reactant concentrations, but it does vary with temperature. K = 1.4 × 10 9 m −1 ·s −1 The rate constant, k, is a proportionality constant that indicates the relationship between the molar concentration of reactants and the rate of a chemical reaction. When the rate law has the special form of. Rate Constant K In A Second Order Reaction.
From www.coursehero.com
[Solved] The secondorder rate constant for the reaction CH3COOC2H5 + OH−... Course Hero Rate Constant K In A Second Order Reaction The rate constant, k, is a proportionality constant that indicates the relationship between the molar concentration of reactants and the rate of a chemical reaction. The rate constant k is independent of the reactant concentrations, but it does vary with temperature. The reaction orders in a rate law describe the. Determine the reaction order and the rate constant. K =. Rate Constant K In A Second Order Reaction.
From www.sliderbase.com
Rate Laws Presentation Chemistry Rate Constant K In A Second Order Reaction Answer second order in ho 2; Determine the reaction order and the rate constant. K = 1.4 × 10 9 m −1 ·s −1 The rate constant, k, is a proportionality constant that indicates the relationship between the molar concentration of reactants and the rate of a chemical reaction. The rate constant k is independent of the reactant concentrations, but. Rate Constant K In A Second Order Reaction.
From www.youtube.com
Intro to Rate Laws, Rate Constants, Reaction Order Chemistry Tutorial YouTube Rate Constant K In A Second Order Reaction A proportionality constant, k, is called the rate constant of the reaction. The rate constant, k, is a proportionality constant that indicates the relationship between the molar concentration of reactants and the rate of a chemical reaction. When the rate law has the special form of a product (or quotient) of powers, f([a], [b],.) = [a] a [b] b [p]. Rate Constant K In A Second Order Reaction.
From www.tpsearchtool.com
How To Calculate The Order Of A Reaction And The Rate Constant Youtube Images Rate Constant K In A Second Order Reaction The rate constant, k, is a proportionality constant that indicates the relationship between the molar concentration of reactants and the rate of a chemical reaction. Determine the reaction order and the rate constant. K = 1.4 × 10 9 m −1 ·s −1 A proportionality constant, k, is called the rate constant of the reaction. When the rate law has. Rate Constant K In A Second Order Reaction.
From www.youtube.com
The rate constant `(K\')` of one reaction is double of the rate constant (K\") of another Rate Constant K In A Second Order Reaction The rate constant k is independent of the reactant concentrations, but it does vary with temperature. The reaction orders in a rate law describe the. Determine the reaction order and the rate constant. K = 1.4 × 10 9 m −1 ·s −1 When the rate law has the special form of a product (or quotient) of powers, f([a], [b],.). Rate Constant K In A Second Order Reaction.
From www.youtube.com
Determine the rate constant (k) for a reaction YouTube Rate Constant K In A Second Order Reaction Answer second order in ho 2; A proportionality constant, k, is called the rate constant of the reaction. The rate constant, k, is a proportionality constant that indicates the relationship between the molar concentration of reactants and the rate of a chemical reaction. When the rate law has the special form of a product (or quotient) of powers, f([a], [b],.). Rate Constant K In A Second Order Reaction.