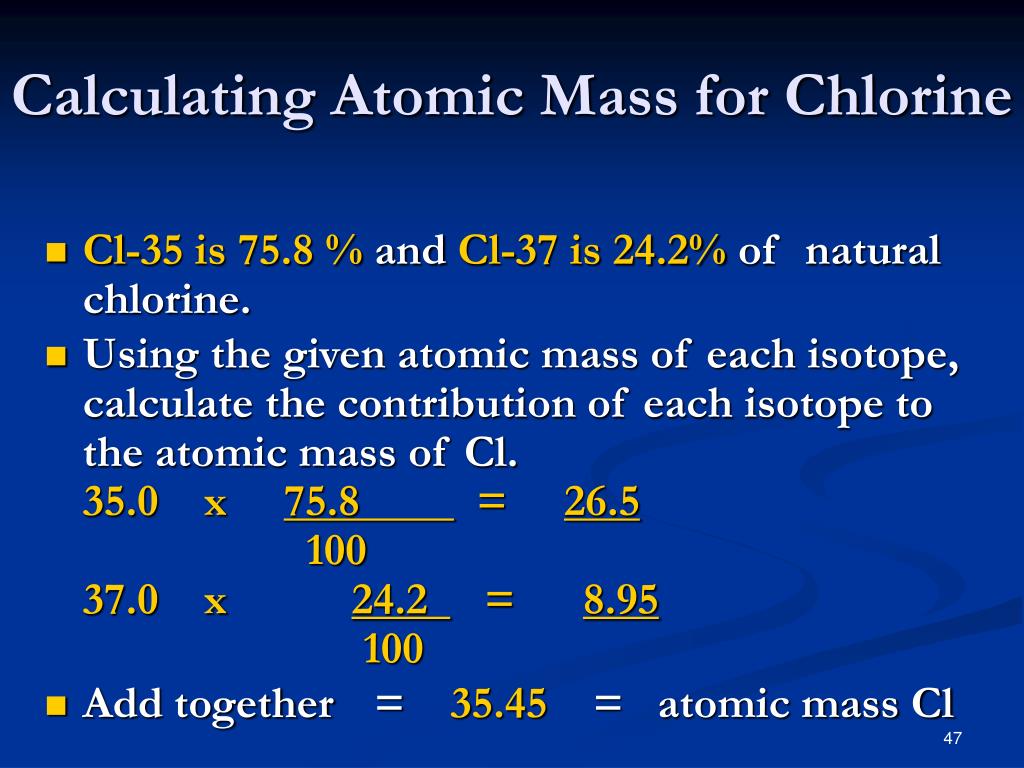
Atomic Weight Of Chlorine Isotopes . This is because chlorine contains two different. For example, the relative atomic mass of chlorine is 35.5 rather than a whole number. Mass numbers of typical isotopes of chlorine are 35; It has an atomic weight of 35.450 and a mass. 53 rows there are two stable isotopes, 35 cl (75.8%) and 37 cl (24.2%), giving chlorine a standard atomic weight of 35.45. Chlorine is the 17th element in the periodic table and has a symbol of cl and atomic number of 17. Isotopes are nuclides that have the same atomic number and are therefore the same element, but differ in the number of neutrons. C) the atomic weight is the average of mass of all isotopes of chlorine atoms and found below the symbol on the periodic table. The atomic number is the number of protons in all chlorine atoms.
from www.slideserve.com
This is because chlorine contains two different. It has an atomic weight of 35.450 and a mass. The atomic number is the number of protons in all chlorine atoms. Chlorine is the 17th element in the periodic table and has a symbol of cl and atomic number of 17. 53 rows there are two stable isotopes, 35 cl (75.8%) and 37 cl (24.2%), giving chlorine a standard atomic weight of 35.45. C) the atomic weight is the average of mass of all isotopes of chlorine atoms and found below the symbol on the periodic table. For example, the relative atomic mass of chlorine is 35.5 rather than a whole number. Mass numbers of typical isotopes of chlorine are 35; Isotopes are nuclides that have the same atomic number and are therefore the same element, but differ in the number of neutrons.
PPT 2.1 Elements and Symbols PowerPoint Presentation, free download
Atomic Weight Of Chlorine Isotopes Chlorine is the 17th element in the periodic table and has a symbol of cl and atomic number of 17. The atomic number is the number of protons in all chlorine atoms. It has an atomic weight of 35.450 and a mass. C) the atomic weight is the average of mass of all isotopes of chlorine atoms and found below the symbol on the periodic table. Chlorine is the 17th element in the periodic table and has a symbol of cl and atomic number of 17. 53 rows there are two stable isotopes, 35 cl (75.8%) and 37 cl (24.2%), giving chlorine a standard atomic weight of 35.45. This is because chlorine contains two different. For example, the relative atomic mass of chlorine is 35.5 rather than a whole number. Mass numbers of typical isotopes of chlorine are 35; Isotopes are nuclides that have the same atomic number and are therefore the same element, but differ in the number of neutrons.
From www.alamy.com
Chlorine (Cl). Diagram of the nuclear composition and electron Atomic Weight Of Chlorine Isotopes For example, the relative atomic mass of chlorine is 35.5 rather than a whole number. Isotopes are nuclides that have the same atomic number and are therefore the same element, but differ in the number of neutrons. C) the atomic weight is the average of mass of all isotopes of chlorine atoms and found below the symbol on the periodic. Atomic Weight Of Chlorine Isotopes.
From www.slideserve.com
PPT KS4 Chemistry PowerPoint Presentation, free download ID5568233 Atomic Weight Of Chlorine Isotopes It has an atomic weight of 35.450 and a mass. For example, the relative atomic mass of chlorine is 35.5 rather than a whole number. Chlorine is the 17th element in the periodic table and has a symbol of cl and atomic number of 17. 53 rows there are two stable isotopes, 35 cl (75.8%) and 37 cl (24.2%), giving. Atomic Weight Of Chlorine Isotopes.
From askfilo.com
Atomic weight of chlorine is 35.5. It has two isotopes of atomic weight 3.. Atomic Weight Of Chlorine Isotopes Chlorine is the 17th element in the periodic table and has a symbol of cl and atomic number of 17. The atomic number is the number of protons in all chlorine atoms. This is because chlorine contains two different. C) the atomic weight is the average of mass of all isotopes of chlorine atoms and found below the symbol on. Atomic Weight Of Chlorine Isotopes.
From www.slideserve.com
PPT 2.1 Elements and Symbols PowerPoint Presentation, free download Atomic Weight Of Chlorine Isotopes Chlorine is the 17th element in the periodic table and has a symbol of cl and atomic number of 17. For example, the relative atomic mass of chlorine is 35.5 rather than a whole number. Isotopes are nuclides that have the same atomic number and are therefore the same element, but differ in the number of neutrons. C) the atomic. Atomic Weight Of Chlorine Isotopes.
From www.youtube.com
Q36&37. Atomic weight of chlorine is 35.5, it has two isotopes of Atomic Weight Of Chlorine Isotopes The atomic number is the number of protons in all chlorine atoms. 53 rows there are two stable isotopes, 35 cl (75.8%) and 37 cl (24.2%), giving chlorine a standard atomic weight of 35.45. Chlorine is the 17th element in the periodic table and has a symbol of cl and atomic number of 17. For example, the relative atomic mass. Atomic Weight Of Chlorine Isotopes.
From www.slideserve.com
PPT Average Atomic Mass PowerPoint Presentation, free download ID Atomic Weight Of Chlorine Isotopes 53 rows there are two stable isotopes, 35 cl (75.8%) and 37 cl (24.2%), giving chlorine a standard atomic weight of 35.45. Chlorine is the 17th element in the periodic table and has a symbol of cl and atomic number of 17. Isotopes are nuclides that have the same atomic number and are therefore the same element, but differ in. Atomic Weight Of Chlorine Isotopes.
From byjus.com
cl 35 and cl 37 are two isotopes of chlorine . if average atomic mass Atomic Weight Of Chlorine Isotopes The atomic number is the number of protons in all chlorine atoms. This is because chlorine contains two different. For example, the relative atomic mass of chlorine is 35.5 rather than a whole number. 53 rows there are two stable isotopes, 35 cl (75.8%) and 37 cl (24.2%), giving chlorine a standard atomic weight of 35.45. Isotopes are nuclides that. Atomic Weight Of Chlorine Isotopes.
From askfilo.com
Atomic weight of chlorine is 35.5. It has two isotopes of atomic weight 3.. Atomic Weight Of Chlorine Isotopes Chlorine is the 17th element in the periodic table and has a symbol of cl and atomic number of 17. 53 rows there are two stable isotopes, 35 cl (75.8%) and 37 cl (24.2%), giving chlorine a standard atomic weight of 35.45. For example, the relative atomic mass of chlorine is 35.5 rather than a whole number. C) the atomic. Atomic Weight Of Chlorine Isotopes.
From periodictable.me
How to Calculate Atomic Mass of Isotopes Archives Dynamic Periodic Atomic Weight Of Chlorine Isotopes Mass numbers of typical isotopes of chlorine are 35; Isotopes are nuclides that have the same atomic number and are therefore the same element, but differ in the number of neutrons. C) the atomic weight is the average of mass of all isotopes of chlorine atoms and found below the symbol on the periodic table. The atomic number is the. Atomic Weight Of Chlorine Isotopes.
From www.doubtnut.com
Atomic weight of chlorine is 35.5 . It has two isotopes of atomic weig Atomic Weight Of Chlorine Isotopes Chlorine is the 17th element in the periodic table and has a symbol of cl and atomic number of 17. This is because chlorine contains two different. The atomic number is the number of protons in all chlorine atoms. It has an atomic weight of 35.450 and a mass. Mass numbers of typical isotopes of chlorine are 35; 53 rows. Atomic Weight Of Chlorine Isotopes.
From www.toppr.com
Atomic mass of chlorine is 35.5. It has two isotopes of atomic mass 35 Atomic Weight Of Chlorine Isotopes Mass numbers of typical isotopes of chlorine are 35; Chlorine is the 17th element in the periodic table and has a symbol of cl and atomic number of 17. Isotopes are nuclides that have the same atomic number and are therefore the same element, but differ in the number of neutrons. For example, the relative atomic mass of chlorine is. Atomic Weight Of Chlorine Isotopes.
From www.slideserve.com
PPT Unit 3 Atomic Structure PowerPoint Presentation, free download Atomic Weight Of Chlorine Isotopes The atomic number is the number of protons in all chlorine atoms. C) the atomic weight is the average of mass of all isotopes of chlorine atoms and found below the symbol on the periodic table. This is because chlorine contains two different. Chlorine is the 17th element in the periodic table and has a symbol of cl and atomic. Atomic Weight Of Chlorine Isotopes.
From www.alamy.com
chlorine chemical element isotopes atomic structure illustration Atomic Weight Of Chlorine Isotopes Chlorine is the 17th element in the periodic table and has a symbol of cl and atomic number of 17. C) the atomic weight is the average of mass of all isotopes of chlorine atoms and found below the symbol on the periodic table. Mass numbers of typical isotopes of chlorine are 35; For example, the relative atomic mass of. Atomic Weight Of Chlorine Isotopes.
From www.nuclear-power.com
Chlorine Atomic Number Atomic Mass Density of Chlorine nuclear Atomic Weight Of Chlorine Isotopes Isotopes are nuclides that have the same atomic number and are therefore the same element, but differ in the number of neutrons. C) the atomic weight is the average of mass of all isotopes of chlorine atoms and found below the symbol on the periodic table. Mass numbers of typical isotopes of chlorine are 35; This is because chlorine contains. Atomic Weight Of Chlorine Isotopes.
From material-properties.org
Chlorine Periodic Table and Atomic Properties Atomic Weight Of Chlorine Isotopes 53 rows there are two stable isotopes, 35 cl (75.8%) and 37 cl (24.2%), giving chlorine a standard atomic weight of 35.45. Chlorine is the 17th element in the periodic table and has a symbol of cl and atomic number of 17. For example, the relative atomic mass of chlorine is 35.5 rather than a whole number. Mass numbers of. Atomic Weight Of Chlorine Isotopes.
From present5.com
Isotopes Atoms of the same element with Atomic Weight Of Chlorine Isotopes It has an atomic weight of 35.450 and a mass. Mass numbers of typical isotopes of chlorine are 35; For example, the relative atomic mass of chlorine is 35.5 rather than a whole number. C) the atomic weight is the average of mass of all isotopes of chlorine atoms and found below the symbol on the periodic table. Isotopes are. Atomic Weight Of Chlorine Isotopes.
From byjus.com
40. Atomic weight of chlorine is 35.5.It has two isotopes of atomic Atomic Weight Of Chlorine Isotopes It has an atomic weight of 35.450 and a mass. For example, the relative atomic mass of chlorine is 35.5 rather than a whole number. Isotopes are nuclides that have the same atomic number and are therefore the same element, but differ in the number of neutrons. C) the atomic weight is the average of mass of all isotopes of. Atomic Weight Of Chlorine Isotopes.
From www.slideserve.com
PPT Isotopes PowerPoint Presentation, free download ID4854680 Atomic Weight Of Chlorine Isotopes For example, the relative atomic mass of chlorine is 35.5 rather than a whole number. C) the atomic weight is the average of mass of all isotopes of chlorine atoms and found below the symbol on the periodic table. Isotopes are nuclides that have the same atomic number and are therefore the same element, but differ in the number of. Atomic Weight Of Chlorine Isotopes.
From avopix.com
Relative Atomic Mass infographic diagram with Royalty Free Stock Atomic Weight Of Chlorine Isotopes 53 rows there are two stable isotopes, 35 cl (75.8%) and 37 cl (24.2%), giving chlorine a standard atomic weight of 35.45. For example, the relative atomic mass of chlorine is 35.5 rather than a whole number. The atomic number is the number of protons in all chlorine atoms. This is because chlorine contains two different. Isotopes are nuclides that. Atomic Weight Of Chlorine Isotopes.
From byjus.com
atomic weight of chlorine 35.5 it has2 isotopes of atomic weight 35 and Atomic Weight Of Chlorine Isotopes It has an atomic weight of 35.450 and a mass. Chlorine is the 17th element in the periodic table and has a symbol of cl and atomic number of 17. For example, the relative atomic mass of chlorine is 35.5 rather than a whole number. The atomic number is the number of protons in all chlorine atoms. Mass numbers of. Atomic Weight Of Chlorine Isotopes.
From askfilo.com
13. 05. Chlorine has 2 isotopes Cl35 and Cl37. Atomic weight of chlorine Atomic Weight Of Chlorine Isotopes It has an atomic weight of 35.450 and a mass. Isotopes are nuclides that have the same atomic number and are therefore the same element, but differ in the number of neutrons. Mass numbers of typical isotopes of chlorine are 35; This is because chlorine contains two different. The atomic number is the number of protons in all chlorine atoms.. Atomic Weight Of Chlorine Isotopes.
From studiousguy.com
Chlorine (Cl) Properties & Uses StudiousGuy Atomic Weight Of Chlorine Isotopes The atomic number is the number of protons in all chlorine atoms. Mass numbers of typical isotopes of chlorine are 35; Isotopes are nuclides that have the same atomic number and are therefore the same element, but differ in the number of neutrons. This is because chlorine contains two different. Chlorine is the 17th element in the periodic table and. Atomic Weight Of Chlorine Isotopes.
From www.doubtnut.com
Chlorine has two isotope ""(17)Cl^(35)Cl^(35) . The atomic weight of Atomic Weight Of Chlorine Isotopes For example, the relative atomic mass of chlorine is 35.5 rather than a whole number. This is because chlorine contains two different. 53 rows there are two stable isotopes, 35 cl (75.8%) and 37 cl (24.2%), giving chlorine a standard atomic weight of 35.45. It has an atomic weight of 35.450 and a mass. Isotopes are nuclides that have the. Atomic Weight Of Chlorine Isotopes.
From greekchlist.weebly.com
Chlorine atomic mass greekchlist Atomic Weight Of Chlorine Isotopes Isotopes are nuclides that have the same atomic number and are therefore the same element, but differ in the number of neutrons. This is because chlorine contains two different. It has an atomic weight of 35.450 and a mass. C) the atomic weight is the average of mass of all isotopes of chlorine atoms and found below the symbol on. Atomic Weight Of Chlorine Isotopes.
From askfilo.com
Average atomic mass of chlorine is 35.5u. It has two isotopes of atomic m.. Atomic Weight Of Chlorine Isotopes This is because chlorine contains two different. Mass numbers of typical isotopes of chlorine are 35; C) the atomic weight is the average of mass of all isotopes of chlorine atoms and found below the symbol on the periodic table. For example, the relative atomic mass of chlorine is 35.5 rather than a whole number. 53 rows there are two. Atomic Weight Of Chlorine Isotopes.
From www.numerade.com
SOLVEDA sample of natural chlorine, has an atomic weight of 35.4527 Atomic Weight Of Chlorine Isotopes C) the atomic weight is the average of mass of all isotopes of chlorine atoms and found below the symbol on the periodic table. Chlorine is the 17th element in the periodic table and has a symbol of cl and atomic number of 17. This is because chlorine contains two different. It has an atomic weight of 35.450 and a. Atomic Weight Of Chlorine Isotopes.
From slideplayer.com
The Periodic Table and the Structure of the Atom ppt download Atomic Weight Of Chlorine Isotopes The atomic number is the number of protons in all chlorine atoms. Isotopes are nuclides that have the same atomic number and are therefore the same element, but differ in the number of neutrons. It has an atomic weight of 35.450 and a mass. Mass numbers of typical isotopes of chlorine are 35; 53 rows there are two stable isotopes,. Atomic Weight Of Chlorine Isotopes.
From www.slideserve.com
PPT Relative atomic mass PowerPoint Presentation ID5933036 Atomic Weight Of Chlorine Isotopes The atomic number is the number of protons in all chlorine atoms. C) the atomic weight is the average of mass of all isotopes of chlorine atoms and found below the symbol on the periodic table. Isotopes are nuclides that have the same atomic number and are therefore the same element, but differ in the number of neutrons. Chlorine is. Atomic Weight Of Chlorine Isotopes.
From slideplayer.com
Average Atomic Mass. ppt download Atomic Weight Of Chlorine Isotopes C) the atomic weight is the average of mass of all isotopes of chlorine atoms and found below the symbol on the periodic table. 53 rows there are two stable isotopes, 35 cl (75.8%) and 37 cl (24.2%), giving chlorine a standard atomic weight of 35.45. This is because chlorine contains two different. For example, the relative atomic mass of. Atomic Weight Of Chlorine Isotopes.
From www.teachoo.com
Isotopes and Isobars Definition, Uses and Difference Teachoo Atomic Weight Of Chlorine Isotopes Chlorine is the 17th element in the periodic table and has a symbol of cl and atomic number of 17. The atomic number is the number of protons in all chlorine atoms. 53 rows there are two stable isotopes, 35 cl (75.8%) and 37 cl (24.2%), giving chlorine a standard atomic weight of 35.45. Mass numbers of typical isotopes of. Atomic Weight Of Chlorine Isotopes.
From slideplayer.com
Chapter 3 Atoms and Elements ppt download Atomic Weight Of Chlorine Isotopes C) the atomic weight is the average of mass of all isotopes of chlorine atoms and found below the symbol on the periodic table. Mass numbers of typical isotopes of chlorine are 35; It has an atomic weight of 35.450 and a mass. For example, the relative atomic mass of chlorine is 35.5 rather than a whole number. Chlorine is. Atomic Weight Of Chlorine Isotopes.
From www.breakingatom.com
Isotopes of Elements Atomic Weight Of Chlorine Isotopes 53 rows there are two stable isotopes, 35 cl (75.8%) and 37 cl (24.2%), giving chlorine a standard atomic weight of 35.45. For example, the relative atomic mass of chlorine is 35.5 rather than a whole number. Isotopes are nuclides that have the same atomic number and are therefore the same element, but differ in the number of neutrons. This. Atomic Weight Of Chlorine Isotopes.
From www.toppr.com
Write the atomic weight of chlorine isotopes. Atomic Weight Of Chlorine Isotopes Isotopes are nuclides that have the same atomic number and are therefore the same element, but differ in the number of neutrons. 53 rows there are two stable isotopes, 35 cl (75.8%) and 37 cl (24.2%), giving chlorine a standard atomic weight of 35.45. Chlorine is the 17th element in the periodic table and has a symbol of cl and. Atomic Weight Of Chlorine Isotopes.
From www.slideserve.com
PPT 1. Atomic Structure PowerPoint Presentation, free download ID Atomic Weight Of Chlorine Isotopes This is because chlorine contains two different. Mass numbers of typical isotopes of chlorine are 35; Chlorine is the 17th element in the periodic table and has a symbol of cl and atomic number of 17. For example, the relative atomic mass of chlorine is 35.5 rather than a whole number. The atomic number is the number of protons in. Atomic Weight Of Chlorine Isotopes.
From www.numerade.com
SOLVED Title Isotopes of Chlorine and Average Atomic Mass Calculation Atomic Weight Of Chlorine Isotopes This is because chlorine contains two different. 53 rows there are two stable isotopes, 35 cl (75.8%) and 37 cl (24.2%), giving chlorine a standard atomic weight of 35.45. Chlorine is the 17th element in the periodic table and has a symbol of cl and atomic number of 17. C) the atomic weight is the average of mass of all. Atomic Weight Of Chlorine Isotopes.