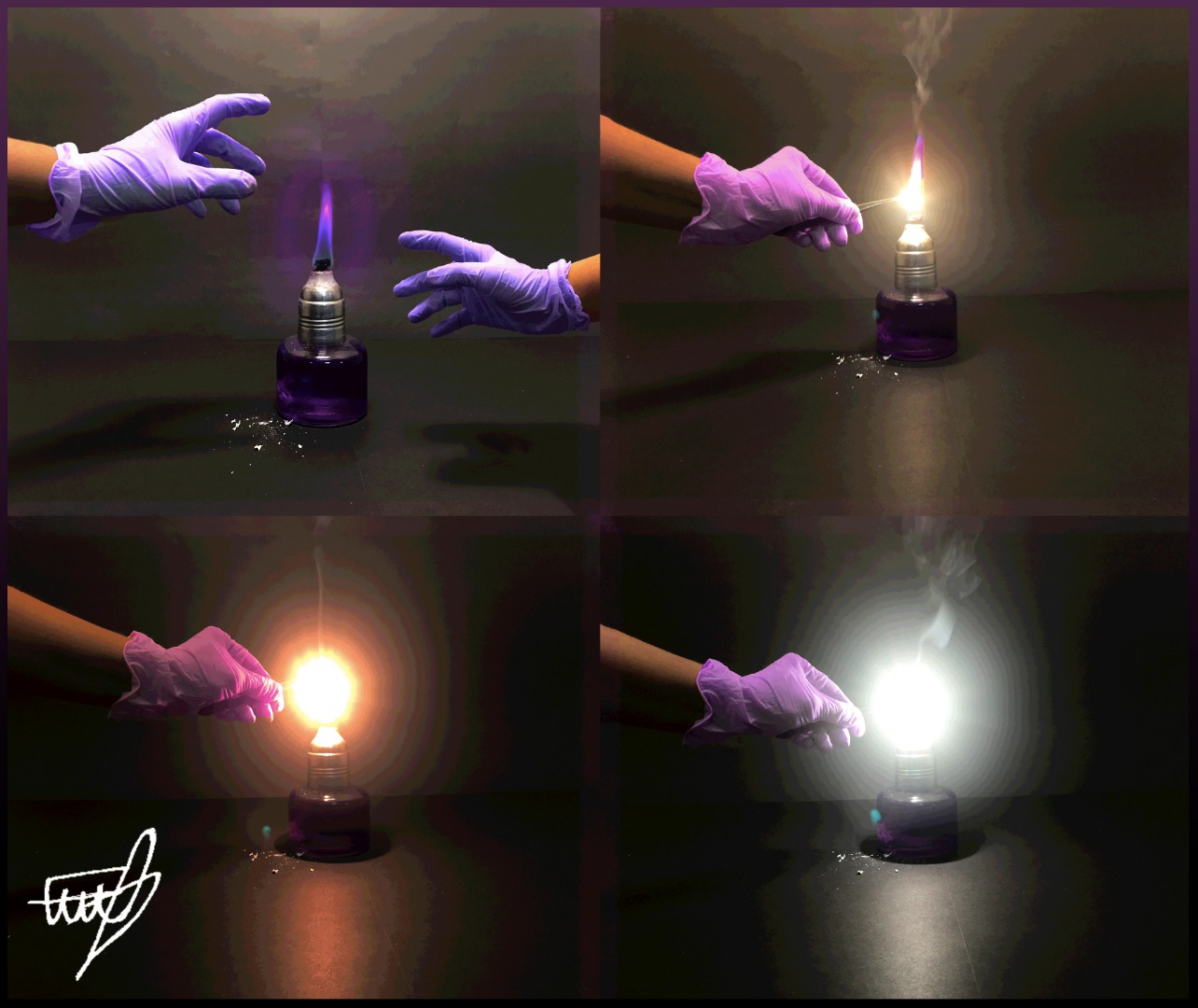
Magnesium Carbonate Flame Test . these flames can be used to produce atomic emmision spectra of the elements combusted. A flame test is relatively quick test for the presence of some elements in a sample. the carbon formed gives off incandescent light in the flame (similar to the cause of the luminosity of a poorly adjusted bunsen flame or a candle flame), and part of. this page describes how to perform a flame test for a range of metal ions, and briefly discusses how the flame color arises. Using known values of emmision spectra, one can perform a flame test on un unknown substance, gather an emmision spectrum from it, and determine which elements are in the unknown substance. to perform flame tests of metal cations in order to observe their characteristic colors, to match the flame colors observed to an. The premise is that heat. the flame test is a qualitative test in analytical chemistry used to help identify the composition of a sample. 50 rows flame test.
from ar.inspiredpencil.com
the carbon formed gives off incandescent light in the flame (similar to the cause of the luminosity of a poorly adjusted bunsen flame or a candle flame), and part of. A flame test is relatively quick test for the presence of some elements in a sample. 50 rows flame test. the flame test is a qualitative test in analytical chemistry used to help identify the composition of a sample. this page describes how to perform a flame test for a range of metal ions, and briefly discusses how the flame color arises. The premise is that heat. to perform flame tests of metal cations in order to observe their characteristic colors, to match the flame colors observed to an. these flames can be used to produce atomic emmision spectra of the elements combusted. Using known values of emmision spectra, one can perform a flame test on un unknown substance, gather an emmision spectrum from it, and determine which elements are in the unknown substance.
Magnesium Flame Test
Magnesium Carbonate Flame Test to perform flame tests of metal cations in order to observe their characteristic colors, to match the flame colors observed to an. the flame test is a qualitative test in analytical chemistry used to help identify the composition of a sample. to perform flame tests of metal cations in order to observe their characteristic colors, to match the flame colors observed to an. The premise is that heat. 50 rows flame test. A flame test is relatively quick test for the presence of some elements in a sample. Using known values of emmision spectra, one can perform a flame test on un unknown substance, gather an emmision spectrum from it, and determine which elements are in the unknown substance. the carbon formed gives off incandescent light in the flame (similar to the cause of the luminosity of a poorly adjusted bunsen flame or a candle flame), and part of. these flames can be used to produce atomic emmision spectra of the elements combusted. this page describes how to perform a flame test for a range of metal ions, and briefly discusses how the flame color arises.
From www.sciencephoto.com
Heating magnesium in a flame Stock Image C001/0851 Science Photo Library Magnesium Carbonate Flame Test these flames can be used to produce atomic emmision spectra of the elements combusted. The premise is that heat. the carbon formed gives off incandescent light in the flame (similar to the cause of the luminosity of a poorly adjusted bunsen flame or a candle flame), and part of. A flame test is relatively quick test for the. Magnesium Carbonate Flame Test.
From sciencenotes.org
Flame Test Colors and Procedure (Chemistry) Magnesium Carbonate Flame Test 50 rows flame test. to perform flame tests of metal cations in order to observe their characteristic colors, to match the flame colors observed to an. the carbon formed gives off incandescent light in the flame (similar to the cause of the luminosity of a poorly adjusted bunsen flame or a candle flame), and part of. A. Magnesium Carbonate Flame Test.
From monroe.com.au
What's The Hottest Color Of Fire?🔥Exploring The Spectrum Of Flame Colors Science And Research Magnesium Carbonate Flame Test to perform flame tests of metal cations in order to observe their characteristic colors, to match the flame colors observed to an. 50 rows flame test. this page describes how to perform a flame test for a range of metal ions, and briefly discusses how the flame color arises. these flames can be used to produce. Magnesium Carbonate Flame Test.
From fphoto.photoshelter.com
science chemistry exothermic reaction magnesium Fundamental Photographs The Art of Science Magnesium Carbonate Flame Test to perform flame tests of metal cations in order to observe their characteristic colors, to match the flame colors observed to an. 50 rows flame test. The premise is that heat. A flame test is relatively quick test for the presence of some elements in a sample. this page describes how to perform a flame test for. Magnesium Carbonate Flame Test.
From www.youtube.com
“Burning magnesium“ experiment from the “Flame“ set YouTube Magnesium Carbonate Flame Test The premise is that heat. Using known values of emmision spectra, one can perform a flame test on un unknown substance, gather an emmision spectrum from it, and determine which elements are in the unknown substance. these flames can be used to produce atomic emmision spectra of the elements combusted. 50 rows flame test. the flame test. Magnesium Carbonate Flame Test.
From saylordotorg.github.io
Periodic Trends and the sBlock Elements Magnesium Carbonate Flame Test A flame test is relatively quick test for the presence of some elements in a sample. these flames can be used to produce atomic emmision spectra of the elements combusted. the carbon formed gives off incandescent light in the flame (similar to the cause of the luminosity of a poorly adjusted bunsen flame or a candle flame), and. Magnesium Carbonate Flame Test.
From ar.inspiredpencil.com
Magnesium Flame Test Magnesium Carbonate Flame Test The premise is that heat. the carbon formed gives off incandescent light in the flame (similar to the cause of the luminosity of a poorly adjusted bunsen flame or a candle flame), and part of. the flame test is a qualitative test in analytical chemistry used to help identify the composition of a sample. to perform flame. Magnesium Carbonate Flame Test.
From www.youtube.com
Magnesium flame test YouTube Magnesium Carbonate Flame Test The premise is that heat. Using known values of emmision spectra, one can perform a flame test on un unknown substance, gather an emmision spectrum from it, and determine which elements are in the unknown substance. 50 rows flame test. the flame test is a qualitative test in analytical chemistry used to help identify the composition of a. Magnesium Carbonate Flame Test.
From eureka.patsnap.com
A kind of micronano basic magnesium carbonate flame retardant and preparation method thereof Magnesium Carbonate Flame Test these flames can be used to produce atomic emmision spectra of the elements combusted. the carbon formed gives off incandescent light in the flame (similar to the cause of the luminosity of a poorly adjusted bunsen flame or a candle flame), and part of. A flame test is relatively quick test for the presence of some elements in. Magnesium Carbonate Flame Test.
From www.slideserve.com
PPT Chemistry in Action PowerPoint Presentation, free download ID6891608 Magnesium Carbonate Flame Test Using known values of emmision spectra, one can perform a flame test on un unknown substance, gather an emmision spectrum from it, and determine which elements are in the unknown substance. the flame test is a qualitative test in analytical chemistry used to help identify the composition of a sample. to perform flame tests of metal cations in. Magnesium Carbonate Flame Test.
From ar.inspiredpencil.com
Lithium Carbonate Flame Test Magnesium Carbonate Flame Test this page describes how to perform a flame test for a range of metal ions, and briefly discusses how the flame color arises. The premise is that heat. the carbon formed gives off incandescent light in the flame (similar to the cause of the luminosity of a poorly adjusted bunsen flame or a candle flame), and part of.. Magnesium Carbonate Flame Test.
From chemistry.com.pk
Metal Ion Flame Test Colours [Infographic] Magnesium Carbonate Flame Test this page describes how to perform a flame test for a range of metal ions, and briefly discusses how the flame color arises. 50 rows flame test. the carbon formed gives off incandescent light in the flame (similar to the cause of the luminosity of a poorly adjusted bunsen flame or a candle flame), and part of.. Magnesium Carbonate Flame Test.
From hightechhigh-faithsdp.weebly.com
Flame Test Lab Faith's DP Magnesium Carbonate Flame Test 50 rows flame test. Using known values of emmision spectra, one can perform a flame test on un unknown substance, gather an emmision spectrum from it, and determine which elements are in the unknown substance. this page describes how to perform a flame test for a range of metal ions, and briefly discusses how the flame color arises.. Magnesium Carbonate Flame Test.
From www.amazingrust.com
Amazing Flame Test Magnesium Carbonate Flame Test these flames can be used to produce atomic emmision spectra of the elements combusted. the flame test is a qualitative test in analytical chemistry used to help identify the composition of a sample. The premise is that heat. 50 rows flame test. this page describes how to perform a flame test for a range of metal. Magnesium Carbonate Flame Test.
From tibetmag.com
Research on the performance of basic magnesium carbonate flame retardant LDPE/EVA Magnesia Magnesium Carbonate Flame Test to perform flame tests of metal cations in order to observe their characteristic colors, to match the flame colors observed to an. these flames can be used to produce atomic emmision spectra of the elements combusted. A flame test is relatively quick test for the presence of some elements in a sample. this page describes how to. Magnesium Carbonate Flame Test.
From worksheetscairpe4d.z21.web.core.windows.net
Procedure Of Flame Test Magnesium Carbonate Flame Test to perform flame tests of metal cations in order to observe their characteristic colors, to match the flame colors observed to an. the carbon formed gives off incandescent light in the flame (similar to the cause of the luminosity of a poorly adjusted bunsen flame or a candle flame), and part of. A flame test is relatively quick. Magnesium Carbonate Flame Test.
From www.youtube.com
Potassium carbonate flame test. YouTube Magnesium Carbonate Flame Test 50 rows flame test. to perform flame tests of metal cations in order to observe their characteristic colors, to match the flame colors observed to an. Using known values of emmision spectra, one can perform a flame test on un unknown substance, gather an emmision spectrum from it, and determine which elements are in the unknown substance. . Magnesium Carbonate Flame Test.
From www.thoughtco.com
Flame Test Colors Photo Gallery Magnesium Carbonate Flame Test the flame test is a qualitative test in analytical chemistry used to help identify the composition of a sample. A flame test is relatively quick test for the presence of some elements in a sample. the carbon formed gives off incandescent light in the flame (similar to the cause of the luminosity of a poorly adjusted bunsen flame. Magnesium Carbonate Flame Test.
From www.youtube.com
Flame Test To identify the metal in a given unknown substance by using flame test. YouTube Magnesium Carbonate Flame Test this page describes how to perform a flame test for a range of metal ions, and briefly discusses how the flame color arises. The premise is that heat. A flame test is relatively quick test for the presence of some elements in a sample. the flame test is a qualitative test in analytical chemistry used to help identify. Magnesium Carbonate Flame Test.
From ar.inspiredpencil.com
Magnesium Flame Test Magnesium Carbonate Flame Test the carbon formed gives off incandescent light in the flame (similar to the cause of the luminosity of a poorly adjusted bunsen flame or a candle flame), and part of. the flame test is a qualitative test in analytical chemistry used to help identify the composition of a sample. these flames can be used to produce atomic. Magnesium Carbonate Flame Test.
From ctdbfredeen.blogspot.com
Lab 11 Flame Test Lab Magnesium Carbonate Flame Test this page describes how to perform a flame test for a range of metal ions, and briefly discusses how the flame color arises. 50 rows flame test. the carbon formed gives off incandescent light in the flame (similar to the cause of the luminosity of a poorly adjusted bunsen flame or a candle flame), and part of.. Magnesium Carbonate Flame Test.
From mungfali.com
Magnesium Flame Test Magnesium Carbonate Flame Test The premise is that heat. Using known values of emmision spectra, one can perform a flame test on un unknown substance, gather an emmision spectrum from it, and determine which elements are in the unknown substance. the carbon formed gives off incandescent light in the flame (similar to the cause of the luminosity of a poorly adjusted bunsen flame. Magnesium Carbonate Flame Test.
From ar.inspiredpencil.com
Magnesium Flame Test Magnesium Carbonate Flame Test The premise is that heat. Using known values of emmision spectra, one can perform a flame test on un unknown substance, gather an emmision spectrum from it, and determine which elements are in the unknown substance. this page describes how to perform a flame test for a range of metal ions, and briefly discusses how the flame color arises.. Magnesium Carbonate Flame Test.
From mavink.com
Magnesium Flame Test Color Magnesium Carbonate Flame Test The premise is that heat. 50 rows flame test. Using known values of emmision spectra, one can perform a flame test on un unknown substance, gather an emmision spectrum from it, and determine which elements are in the unknown substance. A flame test is relatively quick test for the presence of some elements in a sample. to perform. Magnesium Carbonate Flame Test.
From ar.inspiredpencil.com
Magnesium Flame Test Magnesium Carbonate Flame Test The premise is that heat. Using known values of emmision spectra, one can perform a flame test on un unknown substance, gather an emmision spectrum from it, and determine which elements are in the unknown substance. A flame test is relatively quick test for the presence of some elements in a sample. this page describes how to perform a. Magnesium Carbonate Flame Test.
From crimescene2011.blogspot.com
WARNING DO NOT CROSS CSI Flame Tests and Chromatography Magnesium Carbonate Flame Test The premise is that heat. the flame test is a qualitative test in analytical chemistry used to help identify the composition of a sample. these flames can be used to produce atomic emmision spectra of the elements combusted. the carbon formed gives off incandescent light in the flame (similar to the cause of the luminosity of a. Magnesium Carbonate Flame Test.
From ar.inspiredpencil.com
Magnesium Flame Test Magnesium Carbonate Flame Test 50 rows flame test. Using known values of emmision spectra, one can perform a flame test on un unknown substance, gather an emmision spectrum from it, and determine which elements are in the unknown substance. the flame test is a qualitative test in analytical chemistry used to help identify the composition of a sample. The premise is that. Magnesium Carbonate Flame Test.
From mungfali.com
Magnesium Flame Test Magnesium Carbonate Flame Test to perform flame tests of metal cations in order to observe their characteristic colors, to match the flame colors observed to an. the flame test is a qualitative test in analytical chemistry used to help identify the composition of a sample. Using known values of emmision spectra, one can perform a flame test on un unknown substance, gather. Magnesium Carbonate Flame Test.
From sciencenotes.org
Colored Fire Spray Bottles Magnesium Carbonate Flame Test this page describes how to perform a flame test for a range of metal ions, and briefly discusses how the flame color arises. Using known values of emmision spectra, one can perform a flame test on un unknown substance, gather an emmision spectrum from it, and determine which elements are in the unknown substance. A flame test is relatively. Magnesium Carbonate Flame Test.
From fphoto.photoshelter.com
science chemistry flame test Fundamental Photographs The Art of Science Magnesium Carbonate Flame Test 50 rows flame test. Using known values of emmision spectra, one can perform a flame test on un unknown substance, gather an emmision spectrum from it, and determine which elements are in the unknown substance. the carbon formed gives off incandescent light in the flame (similar to the cause of the luminosity of a poorly adjusted bunsen flame. Magnesium Carbonate Flame Test.
From ar.inspiredpencil.com
Flame Test Magnesium Magnesium Carbonate Flame Test Using known values of emmision spectra, one can perform a flame test on un unknown substance, gather an emmision spectrum from it, and determine which elements are in the unknown substance. this page describes how to perform a flame test for a range of metal ions, and briefly discusses how the flame color arises. A flame test is relatively. Magnesium Carbonate Flame Test.
From ar.inspiredpencil.com
Flame Test Calcium Carbonate Magnesium Carbonate Flame Test this page describes how to perform a flame test for a range of metal ions, and briefly discusses how the flame color arises. the flame test is a qualitative test in analytical chemistry used to help identify the composition of a sample. The premise is that heat. to perform flame tests of metal cations in order to. Magnesium Carbonate Flame Test.
From www.sciencephoto.com
Magnesium carbonate precipitate Stock Image C028/0739 Science Photo Library Magnesium Carbonate Flame Test this page describes how to perform a flame test for a range of metal ions, and briefly discusses how the flame color arises. to perform flame tests of metal cations in order to observe their characteristic colors, to match the flame colors observed to an. the flame test is a qualitative test in analytical chemistry used to. Magnesium Carbonate Flame Test.
From www.youtube.com
Flame Tests for Unknowns YouTube Magnesium Carbonate Flame Test to perform flame tests of metal cations in order to observe their characteristic colors, to match the flame colors observed to an. The premise is that heat. 50 rows flame test. Using known values of emmision spectra, one can perform a flame test on un unknown substance, gather an emmision spectrum from it, and determine which elements are. Magnesium Carbonate Flame Test.
From tibetmag.com
Research on basic magnesium carbonate flame retardant epoxy resin Magnesia Supplier Magnesium Carbonate Flame Test A flame test is relatively quick test for the presence of some elements in a sample. this page describes how to perform a flame test for a range of metal ions, and briefly discusses how the flame color arises. these flames can be used to produce atomic emmision spectra of the elements combusted. Using known values of emmision. Magnesium Carbonate Flame Test.