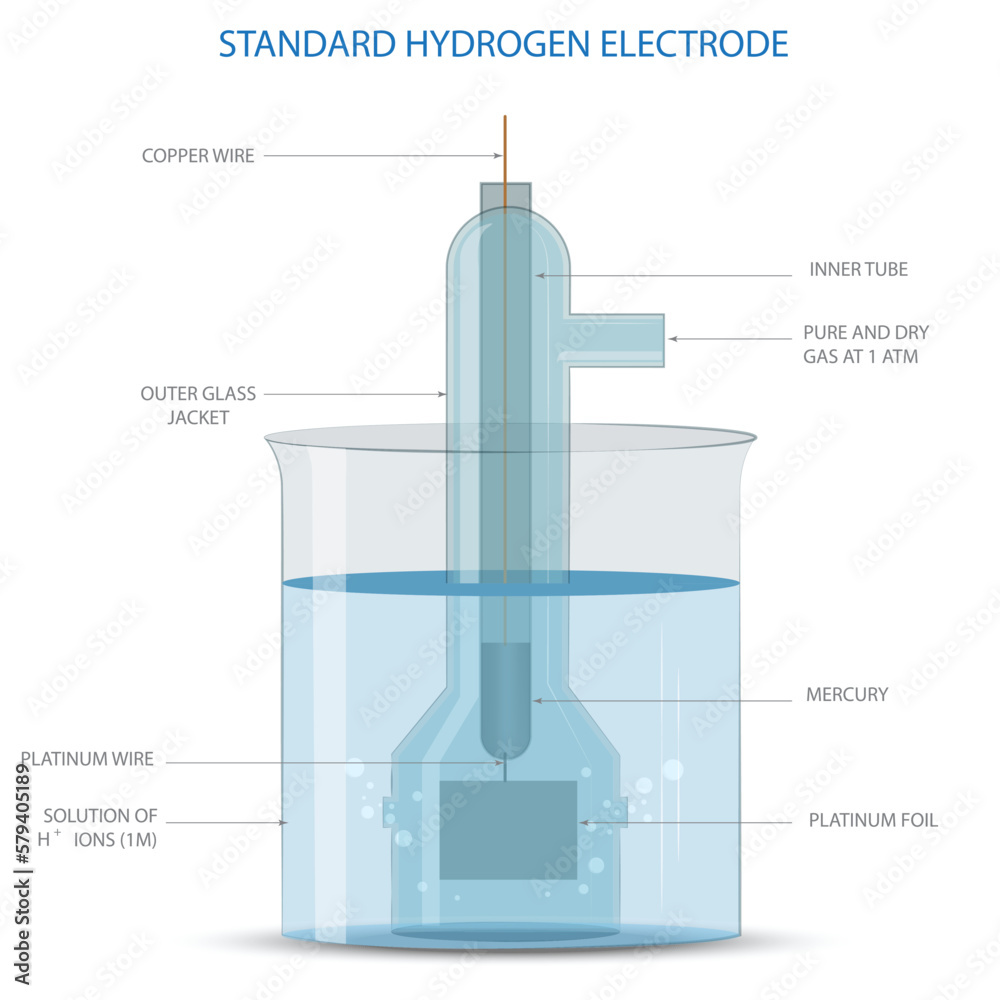
Standard Hydrogen Electrode Simulation . The reference to the simulated potential is typically the standard hydrogen electrode (she), which absolute position is. In this simulation, students select different metals and aqueous solutions to build a galvanic/voltaic cell that generates. The structure of the standard hydrogen electrode is described. Standard hydrogen electrode a platinum electrode is inserted in a solution with a concentration of h+ of 1m, and hydrogen gas at. A platinum electrode is inserted in a solution with a concentration of h + of 1m, and hydrogen gas at 25℃ of 1 atm surrounding the platinum.
from stock.adobe.com
A platinum electrode is inserted in a solution with a concentration of h + of 1m, and hydrogen gas at 25℃ of 1 atm surrounding the platinum. Standard hydrogen electrode a platinum electrode is inserted in a solution with a concentration of h+ of 1m, and hydrogen gas at. The reference to the simulated potential is typically the standard hydrogen electrode (she), which absolute position is. In this simulation, students select different metals and aqueous solutions to build a galvanic/voltaic cell that generates. The structure of the standard hydrogen electrode is described.
Vetor de A Standard Hydrogen Electrode or SHE is an electrode that
Standard Hydrogen Electrode Simulation Standard hydrogen electrode a platinum electrode is inserted in a solution with a concentration of h+ of 1m, and hydrogen gas at. Standard hydrogen electrode a platinum electrode is inserted in a solution with a concentration of h+ of 1m, and hydrogen gas at. The structure of the standard hydrogen electrode is described. The reference to the simulated potential is typically the standard hydrogen electrode (she), which absolute position is. In this simulation, students select different metals and aqueous solutions to build a galvanic/voltaic cell that generates. A platinum electrode is inserted in a solution with a concentration of h + of 1m, and hydrogen gas at 25℃ of 1 atm surrounding the platinum.
From wordwall.net
Standard hydrogen electrode Diagrama con etiquetas Standard Hydrogen Electrode Simulation In this simulation, students select different metals and aqueous solutions to build a galvanic/voltaic cell that generates. Standard hydrogen electrode a platinum electrode is inserted in a solution with a concentration of h+ of 1m, and hydrogen gas at. The reference to the simulated potential is typically the standard hydrogen electrode (she), which absolute position is. A platinum electrode is. Standard Hydrogen Electrode Simulation.
From pubs.acs.org
Standard and Reversible Hydrogen Electrodes Theory, Design, Operation Standard Hydrogen Electrode Simulation A platinum electrode is inserted in a solution with a concentration of h + of 1m, and hydrogen gas at 25℃ of 1 atm surrounding the platinum. In this simulation, students select different metals and aqueous solutions to build a galvanic/voltaic cell that generates. The structure of the standard hydrogen electrode is described. The reference to the simulated potential is. Standard Hydrogen Electrode Simulation.
From byjus.com
Standard Hydrogen Electrode Definition, Construction, and Labelled Standard Hydrogen Electrode Simulation The structure of the standard hydrogen electrode is described. A platinum electrode is inserted in a solution with a concentration of h + of 1m, and hydrogen gas at 25℃ of 1 atm surrounding the platinum. The reference to the simulated potential is typically the standard hydrogen electrode (she), which absolute position is. Standard hydrogen electrode a platinum electrode is. Standard Hydrogen Electrode Simulation.
From www.youtube.com
Standard Hydrogen Electrode Definition ,Construction ,Working Standard Hydrogen Electrode Simulation The reference to the simulated potential is typically the standard hydrogen electrode (she), which absolute position is. A platinum electrode is inserted in a solution with a concentration of h + of 1m, and hydrogen gas at 25℃ of 1 atm surrounding the platinum. The structure of the standard hydrogen electrode is described. In this simulation, students select different metals. Standard Hydrogen Electrode Simulation.
From brainly.in
What is standard hydrogen electrode? How is it prepared? Explain with Standard Hydrogen Electrode Simulation A platinum electrode is inserted in a solution with a concentration of h + of 1m, and hydrogen gas at 25℃ of 1 atm surrounding the platinum. Standard hydrogen electrode a platinum electrode is inserted in a solution with a concentration of h+ of 1m, and hydrogen gas at. In this simulation, students select different metals and aqueous solutions to. Standard Hydrogen Electrode Simulation.
From www.slideserve.com
PPT Chapter 17 Electrochemistry PowerPoint Presentation, free Standard Hydrogen Electrode Simulation Standard hydrogen electrode a platinum electrode is inserted in a solution with a concentration of h+ of 1m, and hydrogen gas at. A platinum electrode is inserted in a solution with a concentration of h + of 1m, and hydrogen gas at 25℃ of 1 atm surrounding the platinum. In this simulation, students select different metals and aqueous solutions to. Standard Hydrogen Electrode Simulation.
From www.slideserve.com
PPT CHEM 160 General Chemistry II Lecture Presentation Standard Hydrogen Electrode Simulation Standard hydrogen electrode a platinum electrode is inserted in a solution with a concentration of h+ of 1m, and hydrogen gas at. The structure of the standard hydrogen electrode is described. The reference to the simulated potential is typically the standard hydrogen electrode (she), which absolute position is. In this simulation, students select different metals and aqueous solutions to build. Standard Hydrogen Electrode Simulation.
From cider.uoregon.edu
Standard Hydrogen Electrode (SHE) Simulation and Animation AACT CIDER Standard Hydrogen Electrode Simulation The reference to the simulated potential is typically the standard hydrogen electrode (she), which absolute position is. Standard hydrogen electrode a platinum electrode is inserted in a solution with a concentration of h+ of 1m, and hydrogen gas at. A platinum electrode is inserted in a solution with a concentration of h + of 1m, and hydrogen gas at 25℃. Standard Hydrogen Electrode Simulation.
From saylordotorg.github.io
Standard Potentials Standard Hydrogen Electrode Simulation Standard hydrogen electrode a platinum electrode is inserted in a solution with a concentration of h+ of 1m, and hydrogen gas at. The structure of the standard hydrogen electrode is described. The reference to the simulated potential is typically the standard hydrogen electrode (she), which absolute position is. A platinum electrode is inserted in a solution with a concentration of. Standard Hydrogen Electrode Simulation.
From www.slideserve.com
PPT Chapter 20 Electrochemistry PowerPoint Presentation, free Standard Hydrogen Electrode Simulation In this simulation, students select different metals and aqueous solutions to build a galvanic/voltaic cell that generates. Standard hydrogen electrode a platinum electrode is inserted in a solution with a concentration of h+ of 1m, and hydrogen gas at. The structure of the standard hydrogen electrode is described. The reference to the simulated potential is typically the standard hydrogen electrode. Standard Hydrogen Electrode Simulation.
From stock.adobe.com
Vetor de A Standard Hydrogen Electrode or SHE is an electrode that Standard Hydrogen Electrode Simulation In this simulation, students select different metals and aqueous solutions to build a galvanic/voltaic cell that generates. The structure of the standard hydrogen electrode is described. Standard hydrogen electrode a platinum electrode is inserted in a solution with a concentration of h+ of 1m, and hydrogen gas at. The reference to the simulated potential is typically the standard hydrogen electrode. Standard Hydrogen Electrode Simulation.
From www.youtube.com
Standard hydrogen Electrode, Normal hydrogen Electrode Standard Hydrogen Electrode Simulation The reference to the simulated potential is typically the standard hydrogen electrode (she), which absolute position is. Standard hydrogen electrode a platinum electrode is inserted in a solution with a concentration of h+ of 1m, and hydrogen gas at. In this simulation, students select different metals and aqueous solutions to build a galvanic/voltaic cell that generates. The structure of the. Standard Hydrogen Electrode Simulation.
From monomole.com
Standard hydrogen electrode Mono Mole Standard Hydrogen Electrode Simulation The reference to the simulated potential is typically the standard hydrogen electrode (she), which absolute position is. A platinum electrode is inserted in a solution with a concentration of h + of 1m, and hydrogen gas at 25℃ of 1 atm surrounding the platinum. Standard hydrogen electrode a platinum electrode is inserted in a solution with a concentration of h+. Standard Hydrogen Electrode Simulation.
From www.shutterstock.com
Diagramme standard d'électrode à hydrogène. Illustration image Standard Hydrogen Electrode Simulation Standard hydrogen electrode a platinum electrode is inserted in a solution with a concentration of h+ of 1m, and hydrogen gas at. A platinum electrode is inserted in a solution with a concentration of h + of 1m, and hydrogen gas at 25℃ of 1 atm surrounding the platinum. The reference to the simulated potential is typically the standard hydrogen. Standard Hydrogen Electrode Simulation.
From question.pandai.org
Standard Electrode Potential Standard Hydrogen Electrode Simulation The structure of the standard hydrogen electrode is described. A platinum electrode is inserted in a solution with a concentration of h + of 1m, and hydrogen gas at 25℃ of 1 atm surrounding the platinum. The reference to the simulated potential is typically the standard hydrogen electrode (she), which absolute position is. In this simulation, students select different metals. Standard Hydrogen Electrode Simulation.
From www.youtube.com
DAT Reaction at Standard Hydrogen Electrode Explained YouTube Standard Hydrogen Electrode Simulation A platinum electrode is inserted in a solution with a concentration of h + of 1m, and hydrogen gas at 25℃ of 1 atm surrounding the platinum. Standard hydrogen electrode a platinum electrode is inserted in a solution with a concentration of h+ of 1m, and hydrogen gas at. The reference to the simulated potential is typically the standard hydrogen. Standard Hydrogen Electrode Simulation.
From www.slideserve.com
PPT Standard hydrogen electrode PowerPoint Presentation, free Standard Hydrogen Electrode Simulation In this simulation, students select different metals and aqueous solutions to build a galvanic/voltaic cell that generates. Standard hydrogen electrode a platinum electrode is inserted in a solution with a concentration of h+ of 1m, and hydrogen gas at. The structure of the standard hydrogen electrode is described. A platinum electrode is inserted in a solution with a concentration of. Standard Hydrogen Electrode Simulation.
From saylordotorg.github.io
Electrochemistry Standard Hydrogen Electrode Simulation The reference to the simulated potential is typically the standard hydrogen electrode (she), which absolute position is. The structure of the standard hydrogen electrode is described. A platinum electrode is inserted in a solution with a concentration of h + of 1m, and hydrogen gas at 25℃ of 1 atm surrounding the platinum. In this simulation, students select different metals. Standard Hydrogen Electrode Simulation.
From www.youtube.com
19.1 Standard hydrogen electrode (HL) YouTube Standard Hydrogen Electrode Simulation A platinum electrode is inserted in a solution with a concentration of h + of 1m, and hydrogen gas at 25℃ of 1 atm surrounding the platinum. The reference to the simulated potential is typically the standard hydrogen electrode (she), which absolute position is. The structure of the standard hydrogen electrode is described. In this simulation, students select different metals. Standard Hydrogen Electrode Simulation.
From www.youtube.com
standard hydrogen electrode YouTube Standard Hydrogen Electrode Simulation Standard hydrogen electrode a platinum electrode is inserted in a solution with a concentration of h+ of 1m, and hydrogen gas at. The reference to the simulated potential is typically the standard hydrogen electrode (she), which absolute position is. A platinum electrode is inserted in a solution with a concentration of h + of 1m, and hydrogen gas at 25℃. Standard Hydrogen Electrode Simulation.
From byjus.com
What is standard hydrogen electrode? Standard Hydrogen Electrode Simulation In this simulation, students select different metals and aqueous solutions to build a galvanic/voltaic cell that generates. The structure of the standard hydrogen electrode is described. A platinum electrode is inserted in a solution with a concentration of h + of 1m, and hydrogen gas at 25℃ of 1 atm surrounding the platinum. The reference to the simulated potential is. Standard Hydrogen Electrode Simulation.
From www.youtube.com
Standard Hydrogen Electrode YouTube Standard Hydrogen Electrode Simulation Standard hydrogen electrode a platinum electrode is inserted in a solution with a concentration of h+ of 1m, and hydrogen gas at. The reference to the simulated potential is typically the standard hydrogen electrode (she), which absolute position is. The structure of the standard hydrogen electrode is described. In this simulation, students select different metals and aqueous solutions to build. Standard Hydrogen Electrode Simulation.
From www.savemyexams.com
The Hydrogen Electrode (HL) HL IB Chemistry Revision Notes 2025 Standard Hydrogen Electrode Simulation In this simulation, students select different metals and aqueous solutions to build a galvanic/voltaic cell that generates. The reference to the simulated potential is typically the standard hydrogen electrode (she), which absolute position is. A platinum electrode is inserted in a solution with a concentration of h + of 1m, and hydrogen gas at 25℃ of 1 atm surrounding the. Standard Hydrogen Electrode Simulation.
From www.youtube.com
Standard Hydrogen Electrode Demonstration YouTube Standard Hydrogen Electrode Simulation A platinum electrode is inserted in a solution with a concentration of h + of 1m, and hydrogen gas at 25℃ of 1 atm surrounding the platinum. The structure of the standard hydrogen electrode is described. The reference to the simulated potential is typically the standard hydrogen electrode (she), which absolute position is. In this simulation, students select different metals. Standard Hydrogen Electrode Simulation.
From loeozfjbx.blob.core.windows.net
Nernst Equation For Standard Hydrogen Electrode at Miguel White blog Standard Hydrogen Electrode Simulation Standard hydrogen electrode a platinum electrode is inserted in a solution with a concentration of h+ of 1m, and hydrogen gas at. The reference to the simulated potential is typically the standard hydrogen electrode (she), which absolute position is. A platinum electrode is inserted in a solution with a concentration of h + of 1m, and hydrogen gas at 25℃. Standard Hydrogen Electrode Simulation.
From cider.uoregon.edu
Standard Hydrogen Electrode (SHE) Simulation and Animation AACT CIDER Standard Hydrogen Electrode Simulation A platinum electrode is inserted in a solution with a concentration of h + of 1m, and hydrogen gas at 25℃ of 1 atm surrounding the platinum. The reference to the simulated potential is typically the standard hydrogen electrode (she), which absolute position is. Standard hydrogen electrode a platinum electrode is inserted in a solution with a concentration of h+. Standard Hydrogen Electrode Simulation.
From pubs.acs.org
Standard and Reversible Hydrogen Electrodes Theory, Design, Operation Standard Hydrogen Electrode Simulation The reference to the simulated potential is typically the standard hydrogen electrode (she), which absolute position is. A platinum electrode is inserted in a solution with a concentration of h + of 1m, and hydrogen gas at 25℃ of 1 atm surrounding the platinum. The structure of the standard hydrogen electrode is described. Standard hydrogen electrode a platinum electrode is. Standard Hydrogen Electrode Simulation.
From chemistnotes.com
Standard hydrogen electrode(SHE) Definition, diagram, application Standard Hydrogen Electrode Simulation In this simulation, students select different metals and aqueous solutions to build a galvanic/voltaic cell that generates. Standard hydrogen electrode a platinum electrode is inserted in a solution with a concentration of h+ of 1m, and hydrogen gas at. The structure of the standard hydrogen electrode is described. The reference to the simulated potential is typically the standard hydrogen electrode. Standard Hydrogen Electrode Simulation.
From www.youtube.com
standard hydrogen electrode (with examples) YouTube Standard Hydrogen Electrode Simulation The structure of the standard hydrogen electrode is described. The reference to the simulated potential is typically the standard hydrogen electrode (she), which absolute position is. A platinum electrode is inserted in a solution with a concentration of h + of 1m, and hydrogen gas at 25℃ of 1 atm surrounding the platinum. Standard hydrogen electrode a platinum electrode is. Standard Hydrogen Electrode Simulation.
From www.youtube.com
Define standard hydrogen electrode SHE and write the reactions that Standard Hydrogen Electrode Simulation Standard hydrogen electrode a platinum electrode is inserted in a solution with a concentration of h+ of 1m, and hydrogen gas at. In this simulation, students select different metals and aqueous solutions to build a galvanic/voltaic cell that generates. The structure of the standard hydrogen electrode is described. The reference to the simulated potential is typically the standard hydrogen electrode. Standard Hydrogen Electrode Simulation.
From www.slideserve.com
PPT Electroanalysis PowerPoint Presentation, free download ID6733376 Standard Hydrogen Electrode Simulation The structure of the standard hydrogen electrode is described. In this simulation, students select different metals and aqueous solutions to build a galvanic/voltaic cell that generates. The reference to the simulated potential is typically the standard hydrogen electrode (she), which absolute position is. Standard hydrogen electrode a platinum electrode is inserted in a solution with a concentration of h+ of. Standard Hydrogen Electrode Simulation.
From solvedlib.com
3 Draw a labeled diagram of standard hydrogen electro… SolvedLib Standard Hydrogen Electrode Simulation The structure of the standard hydrogen electrode is described. The reference to the simulated potential is typically the standard hydrogen electrode (she), which absolute position is. A platinum electrode is inserted in a solution with a concentration of h + of 1m, and hydrogen gas at 25℃ of 1 atm surrounding the platinum. Standard hydrogen electrode a platinum electrode is. Standard Hydrogen Electrode Simulation.
From www.youtube.com
Standard hydrogen electrode SHE Normal hydrogen electrode (NHE Standard Hydrogen Electrode Simulation A platinum electrode is inserted in a solution with a concentration of h + of 1m, and hydrogen gas at 25℃ of 1 atm surrounding the platinum. In this simulation, students select different metals and aqueous solutions to build a galvanic/voltaic cell that generates. The structure of the standard hydrogen electrode is described. Standard hydrogen electrode a platinum electrode is. Standard Hydrogen Electrode Simulation.
From stock.adobe.com
A Standard Hydrogen Electrode (SHE) is an electrode that scientists use Standard Hydrogen Electrode Simulation A platinum electrode is inserted in a solution with a concentration of h + of 1m, and hydrogen gas at 25℃ of 1 atm surrounding the platinum. In this simulation, students select different metals and aqueous solutions to build a galvanic/voltaic cell that generates. Standard hydrogen electrode a platinum electrode is inserted in a solution with a concentration of h+. Standard Hydrogen Electrode Simulation.
From brainly.in
what is standard hydrogen electrode draw Brainly.in Standard Hydrogen Electrode Simulation Standard hydrogen electrode a platinum electrode is inserted in a solution with a concentration of h+ of 1m, and hydrogen gas at. The structure of the standard hydrogen electrode is described. In this simulation, students select different metals and aqueous solutions to build a galvanic/voltaic cell that generates. A platinum electrode is inserted in a solution with a concentration of. Standard Hydrogen Electrode Simulation.