What Is Vapor Pressure Mcat . Vapor pressure is the pressure exerted by the solutions molecules to air molecules. Specific heat, heat of fusion and vaporization example. Simplest way to understand boiling point & vapor pressure. The course focusses on the highest yield material, and serves as a tool to help you get. Vapor pressure represents a state of dynamic equilibrium in which the rate of liquid escaping to gas is equal to the rate of gas. A liquid's vapor pressure is the pressure of the particles just above the liquid. A way to think of it is that increasing vapor pressure. Vapor pressure (or vapour pressure) is the equilibrium pressure of a vapor above its liquid or solid state in a closed container. Courses on khan academy are always 100% free. Makes boiling point, vapor pressure, and what they have to do. Start practicing—and saving your progress—now:. This video is part of the mcat chemistry video course. In this type of closed system, some. They have escaped the liquid phase to the gas phase due to their.
from jackwestin.com
Courses on khan academy are always 100% free. A way to think of it is that increasing vapor pressure. A liquid's vapor pressure is the pressure of the particles just above the liquid. Vapor pressure is the pressure exerted by the solutions molecules to air molecules. The course focusses on the highest yield material, and serves as a tool to help you get. In this type of closed system, some. Makes boiling point, vapor pressure, and what they have to do. Specific heat, heat of fusion and vaporization example. Vapor pressure represents a state of dynamic equilibrium in which the rate of liquid escaping to gas is equal to the rate of gas. Simplest way to understand boiling point & vapor pressure.
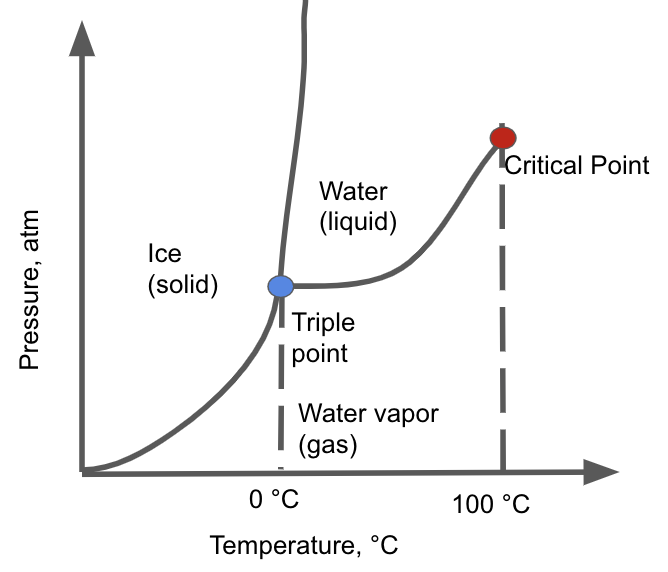
Phase Diagram Pressure And Temperature Energy Changes In Chemical
What Is Vapor Pressure Mcat Vapor pressure (or vapour pressure) is the equilibrium pressure of a vapor above its liquid or solid state in a closed container. Vapor pressure (or vapour pressure) is the equilibrium pressure of a vapor above its liquid or solid state in a closed container. Courses on khan academy are always 100% free. Makes boiling point, vapor pressure, and what they have to do. Vapor pressure represents a state of dynamic equilibrium in which the rate of liquid escaping to gas is equal to the rate of gas. Simplest way to understand boiling point & vapor pressure. In this type of closed system, some. The course focusses on the highest yield material, and serves as a tool to help you get. Start practicing—and saving your progress—now:. Vapor pressure is the pressure exerted by the solutions molecules to air molecules. A way to think of it is that increasing vapor pressure. Specific heat, heat of fusion and vaporization example. This video is part of the mcat chemistry video course. A liquid's vapor pressure is the pressure of the particles just above the liquid. They have escaped the liquid phase to the gas phase due to their.
From jackwestin.com
Phase Diagram Pressure And Temperature Energy Changes In Chemical What Is Vapor Pressure Mcat Start practicing—and saving your progress—now:. Vapor pressure is the pressure exerted by the solutions molecules to air molecules. A liquid's vapor pressure is the pressure of the particles just above the liquid. This video is part of the mcat chemistry video course. They have escaped the liquid phase to the gas phase due to their. Simplest way to understand boiling. What Is Vapor Pressure Mcat.
From www.slideserve.com
PPT Vapour Pressure and Heat PowerPoint Presentation, free download What Is Vapor Pressure Mcat A liquid's vapor pressure is the pressure of the particles just above the liquid. They have escaped the liquid phase to the gas phase due to their. Simplest way to understand boiling point & vapor pressure. Vapor pressure represents a state of dynamic equilibrium in which the rate of liquid escaping to gas is equal to the rate of gas.. What Is Vapor Pressure Mcat.
From www.slideserve.com
PPT Chapter 7 Aerosols, Sprays and Inhalations PowerPoint What Is Vapor Pressure Mcat In this type of closed system, some. Courses on khan academy are always 100% free. A way to think of it is that increasing vapor pressure. Vapor pressure is the pressure exerted by the solutions molecules to air molecules. This video is part of the mcat chemistry video course. Simplest way to understand boiling point & vapor pressure. Specific heat,. What Is Vapor Pressure Mcat.
From www.reddit.com
Lower Boiling Point = more vapor? r/Mcat What Is Vapor Pressure Mcat They have escaped the liquid phase to the gas phase due to their. Courses on khan academy are always 100% free. The course focusses on the highest yield material, and serves as a tool to help you get. This video is part of the mcat chemistry video course. Vapor pressure is the pressure exerted by the solutions molecules to air. What Is Vapor Pressure Mcat.
From sciencenotes.org
Vapor Pressure Definition and How to Calculate It What Is Vapor Pressure Mcat Makes boiling point, vapor pressure, and what they have to do. Courses on khan academy are always 100% free. A liquid's vapor pressure is the pressure of the particles just above the liquid. They have escaped the liquid phase to the gas phase due to their. Specific heat, heat of fusion and vaporization example. The course focusses on the highest. What Is Vapor Pressure Mcat.
From www.slideserve.com
PPT Liquids PowerPoint Presentation, free download ID2237600 What Is Vapor Pressure Mcat Vapor pressure is the pressure exerted by the solutions molecules to air molecules. They have escaped the liquid phase to the gas phase due to their. Courses on khan academy are always 100% free. This video is part of the mcat chemistry video course. Start practicing—and saving your progress—now:. Simplest way to understand boiling point & vapor pressure. Makes boiling. What Is Vapor Pressure Mcat.
From joirgmnzm.blob.core.windows.net
What Is Vapour Pressure Of A Liquid at Rodney Phelps blog What Is Vapor Pressure Mcat Courses on khan academy are always 100% free. This video is part of the mcat chemistry video course. They have escaped the liquid phase to the gas phase due to their. Vapor pressure is the pressure exerted by the solutions molecules to air molecules. Vapor pressure (or vapour pressure) is the equilibrium pressure of a vapor above its liquid or. What Is Vapor Pressure Mcat.
From www.slideserve.com
PPT Vapor Pressure PowerPoint Presentation, free download ID5080574 What Is Vapor Pressure Mcat Courses on khan academy are always 100% free. Vapor pressure is the pressure exerted by the solutions molecules to air molecules. This video is part of the mcat chemistry video course. Vapor pressure (or vapour pressure) is the equilibrium pressure of a vapor above its liquid or solid state in a closed container. In this type of closed system, some.. What Is Vapor Pressure Mcat.
From www.slideserve.com
PPT Vapor Pressure PowerPoint Presentation, free download ID2755893 What Is Vapor Pressure Mcat Makes boiling point, vapor pressure, and what they have to do. They have escaped the liquid phase to the gas phase due to their. This video is part of the mcat chemistry video course. Specific heat, heat of fusion and vaporization example. Courses on khan academy are always 100% free. Start practicing—and saving your progress—now:. Simplest way to understand boiling. What Is Vapor Pressure Mcat.
From www.youtube.com
Vapor Pressure and Boiling YouTube What Is Vapor Pressure Mcat Makes boiling point, vapor pressure, and what they have to do. The course focusses on the highest yield material, and serves as a tool to help you get. Specific heat, heat of fusion and vaporization example. Vapor pressure (or vapour pressure) is the equilibrium pressure of a vapor above its liquid or solid state in a closed container. Vapor pressure. What Is Vapor Pressure Mcat.
From www.chegg.com
Solved The vapor pressure, P, of a certain liquid was What Is Vapor Pressure Mcat Simplest way to understand boiling point & vapor pressure. Vapor pressure represents a state of dynamic equilibrium in which the rate of liquid escaping to gas is equal to the rate of gas. A way to think of it is that increasing vapor pressure. Vapor pressure (or vapour pressure) is the equilibrium pressure of a vapor above its liquid or. What Is Vapor Pressure Mcat.
From www.reddit.com
How to tell which molecule has the highest vapor pressure? Mcat What Is Vapor Pressure Mcat Courses on khan academy are always 100% free. A liquid's vapor pressure is the pressure of the particles just above the liquid. This video is part of the mcat chemistry video course. Start practicing—and saving your progress—now:. Specific heat, heat of fusion and vaporization example. Vapor pressure represents a state of dynamic equilibrium in which the rate of liquid escaping. What Is Vapor Pressure Mcat.
From www.youtube.com
Partial pressure Physical Processes MCAT Khan Academy YouTube What Is Vapor Pressure Mcat Vapor pressure is the pressure exerted by the solutions molecules to air molecules. Makes boiling point, vapor pressure, and what they have to do. Specific heat, heat of fusion and vaporization example. A liquid's vapor pressure is the pressure of the particles just above the liquid. Vapor pressure (or vapour pressure) is the equilibrium pressure of a vapor above its. What Is Vapor Pressure Mcat.
From www.youtube.com
Vapor pressure example Chemistry Khan Academy YouTube What Is Vapor Pressure Mcat Vapor pressure (or vapour pressure) is the equilibrium pressure of a vapor above its liquid or solid state in a closed container. A way to think of it is that increasing vapor pressure. A liquid's vapor pressure is the pressure of the particles just above the liquid. Simplest way to understand boiling point & vapor pressure. Start practicing—and saving your. What Is Vapor Pressure Mcat.
From www.slideserve.com
PPT VAPOR PRESSURE OF WATER PowerPoint Presentation, free download What Is Vapor Pressure Mcat In this type of closed system, some. This video is part of the mcat chemistry video course. The course focusses on the highest yield material, and serves as a tool to help you get. Simplest way to understand boiling point & vapor pressure. Makes boiling point, vapor pressure, and what they have to do. Courses on khan academy are always. What Is Vapor Pressure Mcat.
From saylordotorg.github.io
Vapor Pressure What Is Vapor Pressure Mcat Start practicing—and saving your progress—now:. Simplest way to understand boiling point & vapor pressure. This video is part of the mcat chemistry video course. A liquid's vapor pressure is the pressure of the particles just above the liquid. The course focusses on the highest yield material, and serves as a tool to help you get. Vapor pressure (or vapour pressure). What Is Vapor Pressure Mcat.
From www.youtube.com
2A Osmotic Pressure Video MCAT Math Practice YouTube What Is Vapor Pressure Mcat The course focusses on the highest yield material, and serves as a tool to help you get. They have escaped the liquid phase to the gas phase due to their. This video is part of the mcat chemistry video course. Start practicing—and saving your progress—now:. A way to think of it is that increasing vapor pressure. Simplest way to understand. What Is Vapor Pressure Mcat.
From jackwestin.com
Phase Diagram Pressure And Temperature Energy Changes In Chemical What Is Vapor Pressure Mcat Courses on khan academy are always 100% free. In this type of closed system, some. They have escaped the liquid phase to the gas phase due to their. A liquid's vapor pressure is the pressure of the particles just above the liquid. Simplest way to understand boiling point & vapor pressure. Specific heat, heat of fusion and vaporization example. Vapor. What Is Vapor Pressure Mcat.
From www.ck12.org
Vapor Pressure Overview ( Video ) Chemistry CK12 Foundation What Is Vapor Pressure Mcat Simplest way to understand boiling point & vapor pressure. In this type of closed system, some. Vapor pressure represents a state of dynamic equilibrium in which the rate of liquid escaping to gas is equal to the rate of gas. Vapor pressure is the pressure exerted by the solutions molecules to air molecules. Makes boiling point, vapor pressure, and what. What Is Vapor Pressure Mcat.
From www.researchgate.net
Vapour pressure curve (solid line), critical point (black circle) and What Is Vapor Pressure Mcat Makes boiling point, vapor pressure, and what they have to do. Vapor pressure represents a state of dynamic equilibrium in which the rate of liquid escaping to gas is equal to the rate of gas. Specific heat, heat of fusion and vaporization example. Courses on khan academy are always 100% free. A liquid's vapor pressure is the pressure of the. What Is Vapor Pressure Mcat.
From www.slideshare.net
Vapor pressure and boiling What Is Vapor Pressure Mcat They have escaped the liquid phase to the gas phase due to their. Specific heat, heat of fusion and vaporization example. This video is part of the mcat chemistry video course. Start practicing—and saving your progress—now:. The course focusses on the highest yield material, and serves as a tool to help you get. Vapor pressure (or vapour pressure) is the. What Is Vapor Pressure Mcat.
From medlifemastery.com
Solutions on the MCAT MedLife Mastery What Is Vapor Pressure Mcat Makes boiling point, vapor pressure, and what they have to do. They have escaped the liquid phase to the gas phase due to their. Simplest way to understand boiling point & vapor pressure. A way to think of it is that increasing vapor pressure. Vapor pressure (or vapour pressure) is the equilibrium pressure of a vapor above its liquid or. What Is Vapor Pressure Mcat.
From www.slideserve.com
PPT Chapter 11 Intermolecular Forces, Liquids, and Solids PowerPoint What Is Vapor Pressure Mcat Makes boiling point, vapor pressure, and what they have to do. Courses on khan academy are always 100% free. A way to think of it is that increasing vapor pressure. Vapor pressure represents a state of dynamic equilibrium in which the rate of liquid escaping to gas is equal to the rate of gas. The course focusses on the highest. What Is Vapor Pressure Mcat.
From www.slideserve.com
PPT Vapor Pressure PowerPoint Presentation, free download ID5080574 What Is Vapor Pressure Mcat A liquid's vapor pressure is the pressure of the particles just above the liquid. Courses on khan academy are always 100% free. Specific heat, heat of fusion and vaporization example. In this type of closed system, some. Start practicing—and saving your progress—now:. Vapor pressure is the pressure exerted by the solutions molecules to air molecules. A way to think of. What Is Vapor Pressure Mcat.
From slideplayer.com
Unit 9 Gases. ppt download What Is Vapor Pressure Mcat The course focusses on the highest yield material, and serves as a tool to help you get. Specific heat, heat of fusion and vaporization example. Courses on khan academy are always 100% free. A way to think of it is that increasing vapor pressure. Simplest way to understand boiling point & vapor pressure. Makes boiling point, vapor pressure, and what. What Is Vapor Pressure Mcat.
From www.reddit.com
*Spoiler* EK3 P/S Q29 I thought boiling point increases with vapor What Is Vapor Pressure Mcat Simplest way to understand boiling point & vapor pressure. Vapor pressure (or vapour pressure) is the equilibrium pressure of a vapor above its liquid or solid state in a closed container. Courses on khan academy are always 100% free. Makes boiling point, vapor pressure, and what they have to do. The course focusses on the highest yield material, and serves. What Is Vapor Pressure Mcat.
From www.youtube.com
vapour pressure,definition,unit, factors YouTube What Is Vapor Pressure Mcat Simplest way to understand boiling point & vapor pressure. Vapor pressure (or vapour pressure) is the equilibrium pressure of a vapor above its liquid or solid state in a closed container. Makes boiling point, vapor pressure, and what they have to do. Specific heat, heat of fusion and vaporization example. Start practicing—and saving your progress—now:. Courses on khan academy are. What Is Vapor Pressure Mcat.
From ecoursesonline.iasri.res.in
FM LESSON 3. PROPERTIES OF FLUID What Is Vapor Pressure Mcat Simplest way to understand boiling point & vapor pressure. They have escaped the liquid phase to the gas phase due to their. In this type of closed system, some. Vapor pressure is the pressure exerted by the solutions molecules to air molecules. A liquid's vapor pressure is the pressure of the particles just above the liquid. The course focusses on. What Is Vapor Pressure Mcat.
From www.youtube.com
Evaporation, Vapor Pressure and Boiling YouTube What Is Vapor Pressure Mcat Simplest way to understand boiling point & vapor pressure. This video is part of the mcat chemistry video course. Vapor pressure represents a state of dynamic equilibrium in which the rate of liquid escaping to gas is equal to the rate of gas. A way to think of it is that increasing vapor pressure. Vapor pressure (or vapour pressure) is. What Is Vapor Pressure Mcat.
From www.youtube.com
MCAT® Colligative Properties Vapor Pressure Lowering, Raoult’s Law What Is Vapor Pressure Mcat Simplest way to understand boiling point & vapor pressure. A liquid's vapor pressure is the pressure of the particles just above the liquid. In this type of closed system, some. Vapor pressure is the pressure exerted by the solutions molecules to air molecules. The course focusses on the highest yield material, and serves as a tool to help you get.. What Is Vapor Pressure Mcat.
From www.youtube.com
Heat of Vaporization from Vapor Pressure (Example) YouTube What Is Vapor Pressure Mcat They have escaped the liquid phase to the gas phase due to their. A liquid's vapor pressure is the pressure of the particles just above the liquid. Makes boiling point, vapor pressure, and what they have to do. Courses on khan academy are always 100% free. This video is part of the mcat chemistry video course. In this type of. What Is Vapor Pressure Mcat.
From www.slideserve.com
PPT Vapor Pressure PowerPoint Presentation, free download ID7038735 What Is Vapor Pressure Mcat They have escaped the liquid phase to the gas phase due to their. This video is part of the mcat chemistry video course. The course focusses on the highest yield material, and serves as a tool to help you get. Start practicing—and saving your progress—now:. A liquid's vapor pressure is the pressure of the particles just above the liquid. Courses. What Is Vapor Pressure Mcat.
From study.com
Vapor Pressure Definition, Formula & Examples Lesson What Is Vapor Pressure Mcat They have escaped the liquid phase to the gas phase due to their. The course focusses on the highest yield material, and serves as a tool to help you get. Start practicing—and saving your progress—now:. Courses on khan academy are always 100% free. In this type of closed system, some. Vapor pressure (or vapour pressure) is the equilibrium pressure of. What Is Vapor Pressure Mcat.
From www.youtube.com
Vapour Pressure & Raoults Law Chemistry Class 12 IIT JEE Main What Is Vapor Pressure Mcat Vapor pressure is the pressure exerted by the solutions molecules to air molecules. Courses on khan academy are always 100% free. In this type of closed system, some. The course focusses on the highest yield material, and serves as a tool to help you get. They have escaped the liquid phase to the gas phase due to their. A way. What Is Vapor Pressure Mcat.
From www.youtube.com
What is vapor pressure what is the vapor pressure of liquids What Is Vapor Pressure Mcat In this type of closed system, some. The course focusses on the highest yield material, and serves as a tool to help you get. A liquid's vapor pressure is the pressure of the particles just above the liquid. Start practicing—and saving your progress—now:. Vapor pressure (or vapour pressure) is the equilibrium pressure of a vapor above its liquid or solid. What Is Vapor Pressure Mcat.