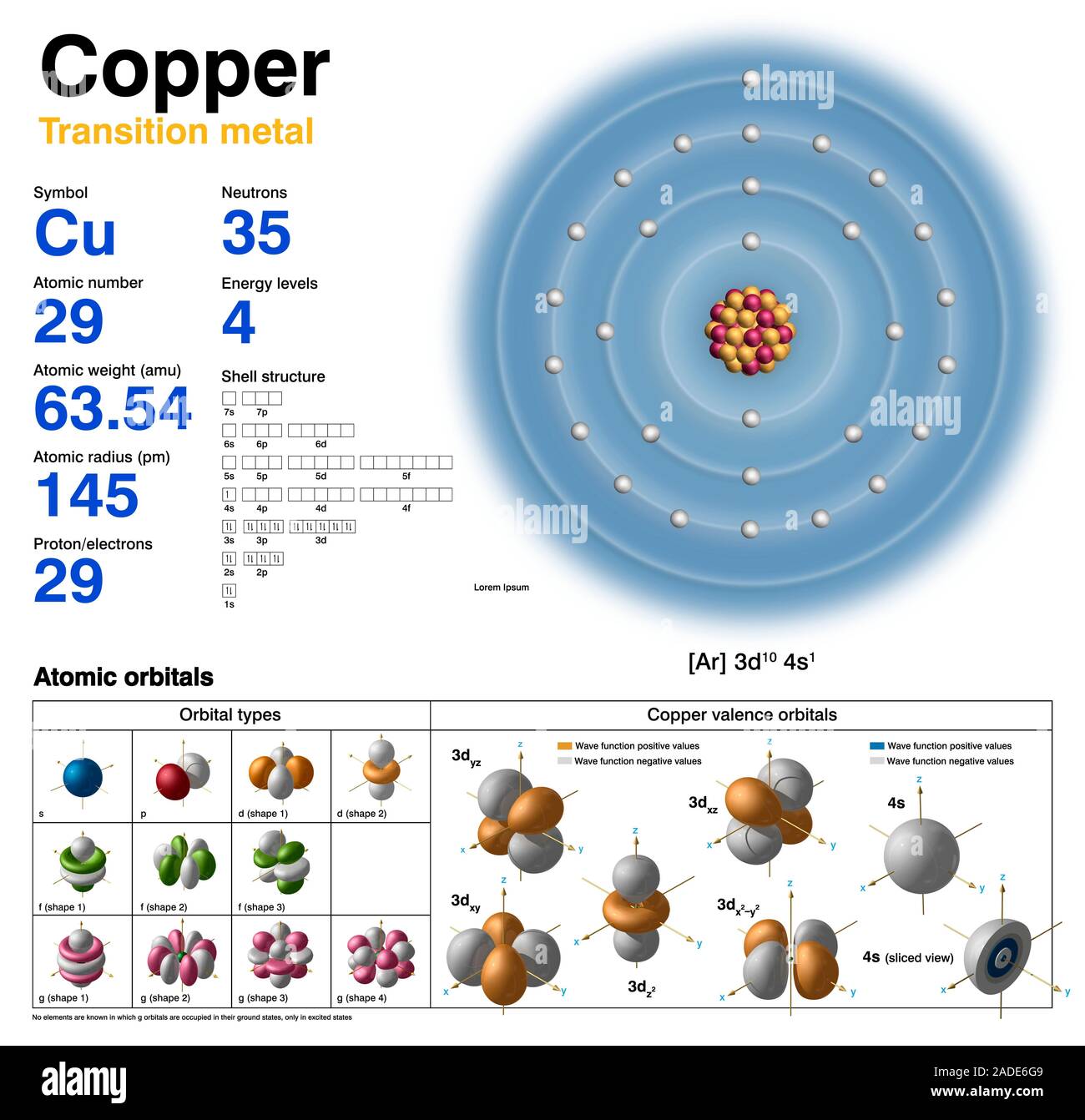
Copper Electron Configuration Form . This means that copper has a completely filled 3d orbital with 10. Copper’s electron configuration is 1s²2s²2p⁶3s²3p⁶4s¹3d¹⁰, deviating from the standard electron filling order due to the. It has 29 electrons distributed in its electron shells. Electron configuration chart of all elements is mentioned in the table below. Learn how to write the electron configuration for copper using the bohr model of orbits and the orbital model of subshells. In order to write the copper electron configuration we first need to know the number of electrons for the cu atom (there are 29 electrons). The electron configuration of copper is [ar] 3d10 4s1. See examples, exceptions, and the order of filling of orbitals. Copper’s electron configuration is 1s² 2s² 2p⁶ 3s² 3p⁶ 4s¹ 3d¹⁰. The shorthand electron configuration (or noble gas configuration) as well as.
from www.alamy.com
The shorthand electron configuration (or noble gas configuration) as well as. See examples, exceptions, and the order of filling of orbitals. Learn how to write the electron configuration for copper using the bohr model of orbits and the orbital model of subshells. In order to write the copper electron configuration we first need to know the number of electrons for the cu atom (there are 29 electrons). It has 29 electrons distributed in its electron shells. Copper’s electron configuration is 1s² 2s² 2p⁶ 3s² 3p⁶ 4s¹ 3d¹⁰. The electron configuration of copper is [ar] 3d10 4s1. Electron configuration chart of all elements is mentioned in the table below. Copper’s electron configuration is 1s²2s²2p⁶3s²3p⁶4s¹3d¹⁰, deviating from the standard electron filling order due to the. This means that copper has a completely filled 3d orbital with 10.
Copper (Cu). Diagram of the nuclear composition, electron configuration
Copper Electron Configuration Form Copper’s electron configuration is 1s² 2s² 2p⁶ 3s² 3p⁶ 4s¹ 3d¹⁰. Copper’s electron configuration is 1s² 2s² 2p⁶ 3s² 3p⁶ 4s¹ 3d¹⁰. Learn how to write the electron configuration for copper using the bohr model of orbits and the orbital model of subshells. This means that copper has a completely filled 3d orbital with 10. It has 29 electrons distributed in its electron shells. The electron configuration of copper is [ar] 3d10 4s1. Electron configuration chart of all elements is mentioned in the table below. The shorthand electron configuration (or noble gas configuration) as well as. In order to write the copper electron configuration we first need to know the number of electrons for the cu atom (there are 29 electrons). See examples, exceptions, and the order of filling of orbitals. Copper’s electron configuration is 1s²2s²2p⁶3s²3p⁶4s¹3d¹⁰, deviating from the standard electron filling order due to the.
From www.dreamstime.com
Electron of the Element Copper Stock Vector Illustration of Copper Electron Configuration Form This means that copper has a completely filled 3d orbital with 10. The electron configuration of copper is [ar] 3d10 4s1. Copper’s electron configuration is 1s² 2s² 2p⁶ 3s² 3p⁶ 4s¹ 3d¹⁰. In order to write the copper electron configuration we first need to know the number of electrons for the cu atom (there are 29 electrons). It has 29. Copper Electron Configuration Form.
From mungfali.com
Electron Configuration Chart Copper Electron Configuration Form In order to write the copper electron configuration we first need to know the number of electrons for the cu atom (there are 29 electrons). This means that copper has a completely filled 3d orbital with 10. Learn how to write the electron configuration for copper using the bohr model of orbits and the orbital model of subshells. The shorthand. Copper Electron Configuration Form.
From www.mooramo.com
Ions of Transition Elements Mooramo Copper Electron Configuration Form It has 29 electrons distributed in its electron shells. In order to write the copper electron configuration we first need to know the number of electrons for the cu atom (there are 29 electrons). Copper’s electron configuration is 1s²2s²2p⁶3s²3p⁶4s¹3d¹⁰, deviating from the standard electron filling order due to the. Electron configuration chart of all elements is mentioned in the table. Copper Electron Configuration Form.
From aliceandallthatjazz.blogspot.com
Electron Configuration Of Copper 1+ worksheet Copper Electron Configuration Form Copper’s electron configuration is 1s² 2s² 2p⁶ 3s² 3p⁶ 4s¹ 3d¹⁰. See examples, exceptions, and the order of filling of orbitals. Copper’s electron configuration is 1s²2s²2p⁶3s²3p⁶4s¹3d¹⁰, deviating from the standard electron filling order due to the. In order to write the copper electron configuration we first need to know the number of electrons for the cu atom (there are 29. Copper Electron Configuration Form.
From electraschematics.com
Understanding the Electron Configuration Diagram for Copper Copper Electron Configuration Form This means that copper has a completely filled 3d orbital with 10. It has 29 electrons distributed in its electron shells. Learn how to write the electron configuration for copper using the bohr model of orbits and the orbital model of subshells. Copper’s electron configuration is 1s² 2s² 2p⁶ 3s² 3p⁶ 4s¹ 3d¹⁰. In order to write the copper electron. Copper Electron Configuration Form.
From ar.inspiredpencil.com
Electron Configuration Of Copper Copper Electron Configuration Form In order to write the copper electron configuration we first need to know the number of electrons for the cu atom (there are 29 electrons). This means that copper has a completely filled 3d orbital with 10. Copper’s electron configuration is 1s²2s²2p⁶3s²3p⁶4s¹3d¹⁰, deviating from the standard electron filling order due to the. The shorthand electron configuration (or noble gas configuration). Copper Electron Configuration Form.
From carisca.github.io
Chromium Electron Configuration Chromium Electrons Neutrons Carisca Copper Electron Configuration Form See examples, exceptions, and the order of filling of orbitals. Learn how to write the electron configuration for copper using the bohr model of orbits and the orbital model of subshells. Copper’s electron configuration is 1s² 2s² 2p⁶ 3s² 3p⁶ 4s¹ 3d¹⁰. It has 29 electrons distributed in its electron shells. Electron configuration chart of all elements is mentioned in. Copper Electron Configuration Form.
From valenceelectrons.com
How to Find the Valence Electrons for Lithium (Li)? Copper Electron Configuration Form In order to write the copper electron configuration we first need to know the number of electrons for the cu atom (there are 29 electrons). The shorthand electron configuration (or noble gas configuration) as well as. It has 29 electrons distributed in its electron shells. This means that copper has a completely filled 3d orbital with 10. Electron configuration chart. Copper Electron Configuration Form.
From learnwithdrscott.com
Electron Configuration Worksheet Easy Hard Science Copper Electron Configuration Form Electron configuration chart of all elements is mentioned in the table below. The shorthand electron configuration (or noble gas configuration) as well as. It has 29 electrons distributed in its electron shells. This means that copper has a completely filled 3d orbital with 10. In order to write the copper electron configuration we first need to know the number of. Copper Electron Configuration Form.
From www.vectorstock.com
Symbol and electron diagram for copper Royalty Free Vector Copper Electron Configuration Form In order to write the copper electron configuration we first need to know the number of electrons for the cu atom (there are 29 electrons). Learn how to write the electron configuration for copper using the bohr model of orbits and the orbital model of subshells. Copper’s electron configuration is 1s²2s²2p⁶3s²3p⁶4s¹3d¹⁰, deviating from the standard electron filling order due to. Copper Electron Configuration Form.
From aliceandallthatjazz.blogspot.com
Electron Configuration Of Copper 1+ worksheet Copper Electron Configuration Form The electron configuration of copper is [ar] 3d10 4s1. Electron configuration chart of all elements is mentioned in the table below. It has 29 electrons distributed in its electron shells. Copper’s electron configuration is 1s² 2s² 2p⁶ 3s² 3p⁶ 4s¹ 3d¹⁰. See examples, exceptions, and the order of filling of orbitals. The shorthand electron configuration (or noble gas configuration) as. Copper Electron Configuration Form.
From ar.inspiredpencil.com
Electron Configuration Of Copper Copper Electron Configuration Form Learn how to write the electron configuration for copper using the bohr model of orbits and the orbital model of subshells. The electron configuration of copper is [ar] 3d10 4s1. Electron configuration chart of all elements is mentioned in the table below. See examples, exceptions, and the order of filling of orbitals. Copper’s electron configuration is 1s² 2s² 2p⁶ 3s². Copper Electron Configuration Form.
From www.youtube.com
Full and Abbreviated Electron Configuration of Copper Cu YouTube Copper Electron Configuration Form See examples, exceptions, and the order of filling of orbitals. Learn how to write the electron configuration for copper using the bohr model of orbits and the orbital model of subshells. It has 29 electrons distributed in its electron shells. The shorthand electron configuration (or noble gas configuration) as well as. This means that copper has a completely filled 3d. Copper Electron Configuration Form.
From valenceelectrons.com
How Many Valence Electrons Does Copper (Cu) Have? Copper Electron Configuration Form Electron configuration chart of all elements is mentioned in the table below. Learn how to write the electron configuration for copper using the bohr model of orbits and the orbital model of subshells. This means that copper has a completely filled 3d orbital with 10. See examples, exceptions, and the order of filling of orbitals. Copper’s electron configuration is 1s². Copper Electron Configuration Form.
From proper-cooking.info
Copper Electron Dot Diagram Copper Electron Configuration Form This means that copper has a completely filled 3d orbital with 10. Copper’s electron configuration is 1s² 2s² 2p⁶ 3s² 3p⁶ 4s¹ 3d¹⁰. Copper’s electron configuration is 1s²2s²2p⁶3s²3p⁶4s¹3d¹⁰, deviating from the standard electron filling order due to the. Learn how to write the electron configuration for copper using the bohr model of orbits and the orbital model of subshells. In. Copper Electron Configuration Form.
From electraschematics.com
Understanding the Electron Configuration Diagram for Copper Copper Electron Configuration Form In order to write the copper electron configuration we first need to know the number of electrons for the cu atom (there are 29 electrons). It has 29 electrons distributed in its electron shells. Copper’s electron configuration is 1s² 2s² 2p⁶ 3s² 3p⁶ 4s¹ 3d¹⁰. See examples, exceptions, and the order of filling of orbitals. Copper’s electron configuration is 1s²2s²2p⁶3s²3p⁶4s¹3d¹⁰,. Copper Electron Configuration Form.
From electraschematics.com
Understanding the Electron Configuration Diagram for Copper Copper Electron Configuration Form Learn how to write the electron configuration for copper using the bohr model of orbits and the orbital model of subshells. Copper’s electron configuration is 1s²2s²2p⁶3s²3p⁶4s¹3d¹⁰, deviating from the standard electron filling order due to the. Copper’s electron configuration is 1s² 2s² 2p⁶ 3s² 3p⁶ 4s¹ 3d¹⁰. Electron configuration chart of all elements is mentioned in the table below. The. Copper Electron Configuration Form.
From www.toppr.com
The electronic configuration of copper (29Cu) is. Copper Electron Configuration Form Electron configuration chart of all elements is mentioned in the table below. The electron configuration of copper is [ar] 3d10 4s1. Copper’s electron configuration is 1s²2s²2p⁶3s²3p⁶4s¹3d¹⁰, deviating from the standard electron filling order due to the. Learn how to write the electron configuration for copper using the bohr model of orbits and the orbital model of subshells. It has 29. Copper Electron Configuration Form.
From www.youtube.com
Give the actual groundstate electron configuration for copper Cu using Copper Electron Configuration Form Copper’s electron configuration is 1s² 2s² 2p⁶ 3s² 3p⁶ 4s¹ 3d¹⁰. The shorthand electron configuration (or noble gas configuration) as well as. Copper’s electron configuration is 1s²2s²2p⁶3s²3p⁶4s¹3d¹⁰, deviating from the standard electron filling order due to the. This means that copper has a completely filled 3d orbital with 10. Learn how to write the electron configuration for copper using the. Copper Electron Configuration Form.
From worksheet-z.netlify.app
Electron Configuration Of Copper 2+ Copper Electron Configuration Form In order to write the copper electron configuration we first need to know the number of electrons for the cu atom (there are 29 electrons). See examples, exceptions, and the order of filling of orbitals. Electron configuration chart of all elements is mentioned in the table below. The electron configuration of copper is [ar] 3d10 4s1. It has 29 electrons. Copper Electron Configuration Form.
From imgtwitter3.blogspot.com
Electron Configuration Of Copper In Ground State Farm house customer Copper Electron Configuration Form It has 29 electrons distributed in its electron shells. The shorthand electron configuration (or noble gas configuration) as well as. Learn how to write the electron configuration for copper using the bohr model of orbits and the orbital model of subshells. See examples, exceptions, and the order of filling of orbitals. Copper’s electron configuration is 1s² 2s² 2p⁶ 3s² 3p⁶. Copper Electron Configuration Form.
From electronconfiguration-11.blogspot.com
79 [TUTORIAL] COPPER I ELECTRON CONFIGURATION with VIDEO + PDF Copper Electron Configuration Form It has 29 electrons distributed in its electron shells. The electron configuration of copper is [ar] 3d10 4s1. Learn how to write the electron configuration for copper using the bohr model of orbits and the orbital model of subshells. In order to write the copper electron configuration we first need to know the number of electrons for the cu atom. Copper Electron Configuration Form.
From www.youtube.com
Electron Configuration for Cu, Cu+, and Cu2+ (Copper and Copper Ions Copper Electron Configuration Form The shorthand electron configuration (or noble gas configuration) as well as. This means that copper has a completely filled 3d orbital with 10. It has 29 electrons distributed in its electron shells. Copper’s electron configuration is 1s²2s²2p⁶3s²3p⁶4s¹3d¹⁰, deviating from the standard electron filling order due to the. Electron configuration chart of all elements is mentioned in the table below. In. Copper Electron Configuration Form.
From general.chemistrysteps.com
Electron Configurations of Ions Chemistry Steps Copper Electron Configuration Form See examples, exceptions, and the order of filling of orbitals. Copper’s electron configuration is 1s²2s²2p⁶3s²3p⁶4s¹3d¹⁰, deviating from the standard electron filling order due to the. Copper’s electron configuration is 1s² 2s² 2p⁶ 3s² 3p⁶ 4s¹ 3d¹⁰. The shorthand electron configuration (or noble gas configuration) as well as. The electron configuration of copper is [ar] 3d10 4s1. Learn how to write. Copper Electron Configuration Form.
From periodictable.me
How To Find A Electron Configuration For Copper Dynamic Periodic Copper Electron Configuration Form Electron configuration chart of all elements is mentioned in the table below. Learn how to write the electron configuration for copper using the bohr model of orbits and the orbital model of subshells. Copper’s electron configuration is 1s² 2s² 2p⁶ 3s² 3p⁶ 4s¹ 3d¹⁰. Copper’s electron configuration is 1s²2s²2p⁶3s²3p⁶4s¹3d¹⁰, deviating from the standard electron filling order due to the. The. Copper Electron Configuration Form.
From www.alamy.com
Copper (Cu). Diagram of the nuclear composition and electron Copper Electron Configuration Form Copper’s electron configuration is 1s²2s²2p⁶3s²3p⁶4s¹3d¹⁰, deviating from the standard electron filling order due to the. It has 29 electrons distributed in its electron shells. The electron configuration of copper is [ar] 3d10 4s1. Electron configuration chart of all elements is mentioned in the table below. The shorthand electron configuration (or noble gas configuration) as well as. In order to write. Copper Electron Configuration Form.
From www.sciencephoto.com
Copper, atomic structure Stock Image C018/3710 Science Photo Library Copper Electron Configuration Form Copper’s electron configuration is 1s² 2s² 2p⁶ 3s² 3p⁶ 4s¹ 3d¹⁰. Learn how to write the electron configuration for copper using the bohr model of orbits and the orbital model of subshells. It has 29 electrons distributed in its electron shells. This means that copper has a completely filled 3d orbital with 10. The electron configuration of copper is [ar]. Copper Electron Configuration Form.
From electraschematics.com
Understanding the Electron Configuration Diagram for Copper Copper Electron Configuration Form Electron configuration chart of all elements is mentioned in the table below. Learn how to write the electron configuration for copper using the bohr model of orbits and the orbital model of subshells. Copper’s electron configuration is 1s²2s²2p⁶3s²3p⁶4s¹3d¹⁰, deviating from the standard electron filling order due to the. It has 29 electrons distributed in its electron shells. The electron configuration. Copper Electron Configuration Form.
From www.alamy.com
Copper (Cu). Diagram of the nuclear composition, electron configuration Copper Electron Configuration Form Copper’s electron configuration is 1s² 2s² 2p⁶ 3s² 3p⁶ 4s¹ 3d¹⁰. In order to write the copper electron configuration we first need to know the number of electrons for the cu atom (there are 29 electrons). The shorthand electron configuration (or noble gas configuration) as well as. Copper’s electron configuration is 1s²2s²2p⁶3s²3p⁶4s¹3d¹⁰, deviating from the standard electron filling order due. Copper Electron Configuration Form.
From www.youtube.com
Copper Electron Configuration Organic Chemistry Examples YouTube Copper Electron Configuration Form Electron configuration chart of all elements is mentioned in the table below. The shorthand electron configuration (or noble gas configuration) as well as. Copper’s electron configuration is 1s² 2s² 2p⁶ 3s² 3p⁶ 4s¹ 3d¹⁰. Copper’s electron configuration is 1s²2s²2p⁶3s²3p⁶4s¹3d¹⁰, deviating from the standard electron filling order due to the. It has 29 electrons distributed in its electron shells. The electron. Copper Electron Configuration Form.
From valenceelectrons.com
Electron Configuration for Copper (Cu, Cu+, Cu2+) Copper Electron Configuration Form It has 29 electrons distributed in its electron shells. Copper’s electron configuration is 1s²2s²2p⁶3s²3p⁶4s¹3d¹⁰, deviating from the standard electron filling order due to the. Electron configuration chart of all elements is mentioned in the table below. This means that copper has a completely filled 3d orbital with 10. Copper’s electron configuration is 1s² 2s² 2p⁶ 3s² 3p⁶ 4s¹ 3d¹⁰. In. Copper Electron Configuration Form.
From valenceelectrons.com
Copper(Cu) electron configuration and orbital diagram Copper Electron Configuration Form In order to write the copper electron configuration we first need to know the number of electrons for the cu atom (there are 29 electrons). Copper’s electron configuration is 1s² 2s² 2p⁶ 3s² 3p⁶ 4s¹ 3d¹⁰. Electron configuration chart of all elements is mentioned in the table below. The shorthand electron configuration (or noble gas configuration) as well as. It. Copper Electron Configuration Form.
From www.coursehero.com
[Solved] 15. Fill in the electron configuration diagram for the copper Copper Electron Configuration Form Copper’s electron configuration is 1s²2s²2p⁶3s²3p⁶4s¹3d¹⁰, deviating from the standard electron filling order due to the. The electron configuration of copper is [ar] 3d10 4s1. Copper’s electron configuration is 1s² 2s² 2p⁶ 3s² 3p⁶ 4s¹ 3d¹⁰. Learn how to write the electron configuration for copper using the bohr model of orbits and the orbital model of subshells. It has 29 electrons. Copper Electron Configuration Form.
From ar.inspiredpencil.com
Electron Configuration Of Copper Copper Electron Configuration Form In order to write the copper electron configuration we first need to know the number of electrons for the cu atom (there are 29 electrons). Copper’s electron configuration is 1s² 2s² 2p⁶ 3s² 3p⁶ 4s¹ 3d¹⁰. The shorthand electron configuration (or noble gas configuration) as well as. Learn how to write the electron configuration for copper using the bohr model. Copper Electron Configuration Form.
From aliceandallthatjazz.blogspot.com
Electron Configuration Of Copper 1+ worksheet Copper Electron Configuration Form In order to write the copper electron configuration we first need to know the number of electrons for the cu atom (there are 29 electrons). The shorthand electron configuration (or noble gas configuration) as well as. Electron configuration chart of all elements is mentioned in the table below. Copper’s electron configuration is 1s² 2s² 2p⁶ 3s² 3p⁶ 4s¹ 3d¹⁰. The. Copper Electron Configuration Form.