High Vs Low Pka . Pka is the negative log of the acid dissociation constant of a solution. Pka is the negative logarithm of the acid dissociation constant of a solution. Learn how to compare the acidities of different compounds using the pka parameter, which measures how tightly a proton is held by. Learn how to calculate the acid ionization constant (ka) and the acid dissociation constant (pka) for weak acids and their. It measures the strength of an acid by how tightly. A low ph value indicates acidity, a ph of 7 is neutral, and a high ph value indicates alkalinity. Learn how to quantify the acid strength of organic compounds using pka values and periodic trends. Learn about the quantitative measure of acid strength in solution, its theoretical background, definitions, applications, and values for. Learn how to calculate pka and ph and. It indicates the strength of an acid and can be used to select buffers. The ph value can tell you whether you're dealing with an acid or a base,.
from www.masterorganicchemistry.com
It indicates the strength of an acid and can be used to select buffers. Pka is the negative logarithm of the acid dissociation constant of a solution. It measures the strength of an acid by how tightly. The ph value can tell you whether you're dealing with an acid or a base,. Learn how to calculate pka and ph and. Learn about the quantitative measure of acid strength in solution, its theoretical background, definitions, applications, and values for. Learn how to quantify the acid strength of organic compounds using pka values and periodic trends. A low ph value indicates acidity, a ph of 7 is neutral, and a high ph value indicates alkalinity. Pka is the negative log of the acid dissociation constant of a solution. Learn how to compare the acidities of different compounds using the pka parameter, which measures how tightly a proton is held by.
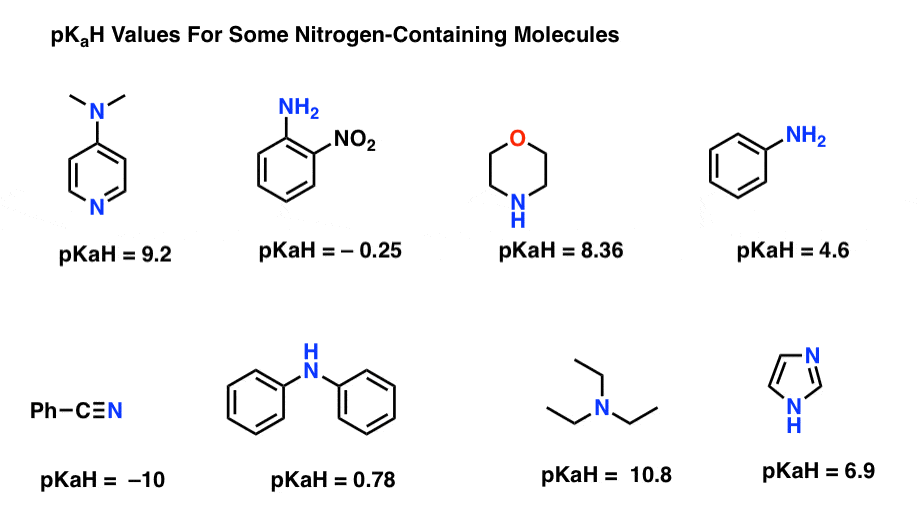
Amine Basicity Is Measued By The pKa Of Its Conjugate Acid (pKaH)
High Vs Low Pka Learn how to calculate the acid ionization constant (ka) and the acid dissociation constant (pka) for weak acids and their. Learn how to quantify the acid strength of organic compounds using pka values and periodic trends. Pka is the negative log of the acid dissociation constant of a solution. The ph value can tell you whether you're dealing with an acid or a base,. Pka is the negative logarithm of the acid dissociation constant of a solution. Learn how to calculate pka and ph and. It measures the strength of an acid by how tightly. Learn how to compare the acidities of different compounds using the pka parameter, which measures how tightly a proton is held by. A low ph value indicates acidity, a ph of 7 is neutral, and a high ph value indicates alkalinity. Learn how to calculate the acid ionization constant (ka) and the acid dissociation constant (pka) for weak acids and their. It indicates the strength of an acid and can be used to select buffers. Learn about the quantitative measure of acid strength in solution, its theoretical background, definitions, applications, and values for.
From www.masterorganicchemistry.com
How To Use a pKa Table High Vs Low Pka Learn about the quantitative measure of acid strength in solution, its theoretical background, definitions, applications, and values for. Learn how to quantify the acid strength of organic compounds using pka values and periodic trends. A low ph value indicates acidity, a ph of 7 is neutral, and a high ph value indicates alkalinity. The ph value can tell you whether. High Vs Low Pka.
From www.researchgate.net
pKa vs pH relation for TC and HAP Predicted fraction of available High Vs Low Pka The ph value can tell you whether you're dealing with an acid or a base,. Learn about the quantitative measure of acid strength in solution, its theoretical background, definitions, applications, and values for. Learn how to compare the acidities of different compounds using the pka parameter, which measures how tightly a proton is held by. Learn how to quantify the. High Vs Low Pka.
From pilotinstitute.com
High vs. LowPressure Systems Explained Pilot Institute High Vs Low Pka Pka is the negative logarithm of the acid dissociation constant of a solution. It indicates the strength of an acid and can be used to select buffers. Learn how to compare the acidities of different compounds using the pka parameter, which measures how tightly a proton is held by. Learn about the quantitative measure of acid strength in solution, its. High Vs Low Pka.
From www.masterorganicchemistry.com
Amine Basicity Is Measued By The pKa Of Its Conjugate Acid (pKaH) High Vs Low Pka Learn how to calculate pka and ph and. Learn how to compare the acidities of different compounds using the pka parameter, which measures how tightly a proton is held by. Pka is the negative logarithm of the acid dissociation constant of a solution. Learn about the quantitative measure of acid strength in solution, its theoretical background, definitions, applications, and values. High Vs Low Pka.
From doktormike.gitlab.io
Michael Green LogP, LogD, pKa and LogS A Physicists guide to basic High Vs Low Pka It indicates the strength of an acid and can be used to select buffers. Learn how to calculate pka and ph and. Learn how to compare the acidities of different compounds using the pka parameter, which measures how tightly a proton is held by. Learn how to quantify the acid strength of organic compounds using pka values and periodic trends.. High Vs Low Pka.
From gpatindia.com
Amino Acids Structure Classification Functions and Properties and High Vs Low Pka Learn how to compare the acidities of different compounds using the pka parameter, which measures how tightly a proton is held by. The ph value can tell you whether you're dealing with an acid or a base,. Learn how to quantify the acid strength of organic compounds using pka values and periodic trends. It indicates the strength of an acid. High Vs Low Pka.
From kunduz.com
What is pKa Definition, Calculation, List of pKa Values, Relationship High Vs Low Pka Learn how to calculate pka and ph and. Learn how to compare the acidities of different compounds using the pka parameter, which measures how tightly a proton is held by. A low ph value indicates acidity, a ph of 7 is neutral, and a high ph value indicates alkalinity. It indicates the strength of an acid and can be used. High Vs Low Pka.
From petersonhinse1964.blogspot.com
How To Get Pka From Ka Peterson Hinse1964 High Vs Low Pka The ph value can tell you whether you're dealing with an acid or a base,. A low ph value indicates acidity, a ph of 7 is neutral, and a high ph value indicates alkalinity. Pka is the negative log of the acid dissociation constant of a solution. Learn about the quantitative measure of acid strength in solution, its theoretical background,. High Vs Low Pka.
From www.masterorganicchemistry.com
How To Use a pKa Table High Vs Low Pka Learn how to quantify the acid strength of organic compounds using pka values and periodic trends. Pka is the negative logarithm of the acid dissociation constant of a solution. Learn how to compare the acidities of different compounds using the pka parameter, which measures how tightly a proton is held by. Learn how to calculate pka and ph and. A. High Vs Low Pka.
From www.gbu-presnenskij.ru
Difference Between PKa And PH Definition, Values,, 52 OFF High Vs Low Pka Pka is the negative log of the acid dissociation constant of a solution. Pka is the negative logarithm of the acid dissociation constant of a solution. A low ph value indicates acidity, a ph of 7 is neutral, and a high ph value indicates alkalinity. Learn how to calculate pka and ph and. The ph value can tell you whether. High Vs Low Pka.
From kpu.pressbooks.pub
3.3 pKa of Organic Acids and Application of pKa to Predict AcidBase High Vs Low Pka Learn how to calculate the acid ionization constant (ka) and the acid dissociation constant (pka) for weak acids and their. Learn how to compare the acidities of different compounds using the pka parameter, which measures how tightly a proton is held by. Pka is the negative log of the acid dissociation constant of a solution. The ph value can tell. High Vs Low Pka.
From www.youtube.com
pKa, Ka, and Acid Strength YouTube High Vs Low Pka Learn about the quantitative measure of acid strength in solution, its theoretical background, definitions, applications, and values for. Learn how to calculate pka and ph and. Pka is the negative log of the acid dissociation constant of a solution. It indicates the strength of an acid and can be used to select buffers. Learn how to compare the acidities of. High Vs Low Pka.
From www.youtube.com
pH vs pKa vs pI vs pKR and terminology around basic & acidic amino High Vs Low Pka Learn how to calculate the acid ionization constant (ka) and the acid dissociation constant (pka) for weak acids and their. The ph value can tell you whether you're dealing with an acid or a base,. A low ph value indicates acidity, a ph of 7 is neutral, and a high ph value indicates alkalinity. Learn how to calculate pka and. High Vs Low Pka.
From sciencenotes.org
What Is pKa in Chemistry? Acid Dissociation Constant High Vs Low Pka It indicates the strength of an acid and can be used to select buffers. Learn how to calculate pka and ph and. Pka is the negative logarithm of the acid dissociation constant of a solution. Learn how to compare the acidities of different compounds using the pka parameter, which measures how tightly a proton is held by. Learn how to. High Vs Low Pka.
From www.thoughtco.com
pKa Definition in Chemistry High Vs Low Pka Pka is the negative log of the acid dissociation constant of a solution. Learn how to calculate pka and ph and. Learn how to calculate the acid ionization constant (ka) and the acid dissociation constant (pka) for weak acids and their. Learn how to compare the acidities of different compounds using the pka parameter, which measures how tightly a proton. High Vs Low Pka.
From www.showme.com
ShowMe pka High Vs Low Pka Learn how to compare the acidities of different compounds using the pka parameter, which measures how tightly a proton is held by. A low ph value indicates acidity, a ph of 7 is neutral, and a high ph value indicates alkalinity. It measures the strength of an acid by how tightly. It indicates the strength of an acid and can. High Vs Low Pka.
From bitesizebio.com
What Is pH? What Are pKa and pI? 3 Key Units. 1 Savvy Guide High Vs Low Pka The ph value can tell you whether you're dealing with an acid or a base,. Learn how to calculate the acid ionization constant (ka) and the acid dissociation constant (pka) for weak acids and their. Learn about the quantitative measure of acid strength in solution, its theoretical background, definitions, applications, and values for. Learn how to compare the acidities of. High Vs Low Pka.
From www.chegg.com
Solved Rank the following carboxylic acids from lowest to High Vs Low Pka Pka is the negative log of the acid dissociation constant of a solution. It measures the strength of an acid by how tightly. It indicates the strength of an acid and can be used to select buffers. Pka is the negative logarithm of the acid dissociation constant of a solution. Learn how to quantify the acid strength of organic compounds. High Vs Low Pka.
From www.researchgate.net
Amino Acid pKa and corresponding protonation states at low, medium, and High Vs Low Pka It measures the strength of an acid by how tightly. It indicates the strength of an acid and can be used to select buffers. Learn how to compare the acidities of different compounds using the pka parameter, which measures how tightly a proton is held by. Learn how to calculate pka and ph and. A low ph value indicates acidity,. High Vs Low Pka.
From www.numerade.com
SOLVEDExplain the low pKa for aromatic amines and amide functional High Vs Low Pka The ph value can tell you whether you're dealing with an acid or a base,. Learn how to quantify the acid strength of organic compounds using pka values and periodic trends. Pka is the negative logarithm of the acid dissociation constant of a solution. A low ph value indicates acidity, a ph of 7 is neutral, and a high ph. High Vs Low Pka.
From www.organicchemistrytutor.com
AcidBase Equilibrium Part 1 How to Use the pKa Table — Organic High Vs Low Pka Pka is the negative log of the acid dissociation constant of a solution. Learn how to calculate pka and ph and. Learn how to calculate the acid ionization constant (ka) and the acid dissociation constant (pka) for weak acids and their. The ph value can tell you whether you're dealing with an acid or a base,. It indicates the strength. High Vs Low Pka.
From pediaa.com
Difference Between pKa and pH High Vs Low Pka Pka is the negative log of the acid dissociation constant of a solution. It measures the strength of an acid by how tightly. A low ph value indicates acidity, a ph of 7 is neutral, and a high ph value indicates alkalinity. Learn about the quantitative measure of acid strength in solution, its theoretical background, definitions, applications, and values for.. High Vs Low Pka.
From www.masterorganicchemistry.com
How To Use a pKa Table High Vs Low Pka Learn how to quantify the acid strength of organic compounds using pka values and periodic trends. The ph value can tell you whether you're dealing with an acid or a base,. Learn about the quantitative measure of acid strength in solution, its theoretical background, definitions, applications, and values for. Pka is the negative log of the acid dissociation constant of. High Vs Low Pka.
From www.researchgate.net
Measured pKa values for selected BCAA/FBCAA pairs. pKa acid 5 High Vs Low Pka Pka is the negative log of the acid dissociation constant of a solution. Learn how to compare the acidities of different compounds using the pka parameter, which measures how tightly a proton is held by. The ph value can tell you whether you're dealing with an acid or a base,. It indicates the strength of an acid and can be. High Vs Low Pka.
From www.masterorganicchemistry.com
How To Use a pKa Table High Vs Low Pka Pka is the negative logarithm of the acid dissociation constant of a solution. Learn how to compare the acidities of different compounds using the pka parameter, which measures how tightly a proton is held by. The ph value can tell you whether you're dealing with an acid or a base,. Learn how to quantify the acid strength of organic compounds. High Vs Low Pka.
From www.slideserve.com
PPT pKa concepts PowerPoint Presentation, free download ID6594110 High Vs Low Pka Learn about the quantitative measure of acid strength in solution, its theoretical background, definitions, applications, and values for. Learn how to quantify the acid strength of organic compounds using pka values and periodic trends. It measures the strength of an acid by how tightly. Learn how to calculate pka and ph and. It indicates the strength of an acid and. High Vs Low Pka.
From www.youtube.com
Aromaticity and Acidity and Basicity Analyzing the relative sizes of High Vs Low Pka Learn how to calculate pka and ph and. Pka is the negative log of the acid dissociation constant of a solution. Learn how to calculate the acid ionization constant (ka) and the acid dissociation constant (pka) for weak acids and their. Pka is the negative logarithm of the acid dissociation constant of a solution. Learn about the quantitative measure of. High Vs Low Pka.
From www.reddit.com
Why do acidbase reactions always go from higher acidity to lower High Vs Low Pka Learn how to calculate the acid ionization constant (ka) and the acid dissociation constant (pka) for weak acids and their. Pka is the negative log of the acid dissociation constant of a solution. It indicates the strength of an acid and can be used to select buffers. Learn about the quantitative measure of acid strength in solution, its theoretical background,. High Vs Low Pka.
From www.masterorganicchemistry.com
Amine Basicity Is Measued By The pKa Of Its Conjugate Acid (pKaH) High Vs Low Pka Pka is the negative log of the acid dissociation constant of a solution. Pka is the negative logarithm of the acid dissociation constant of a solution. A low ph value indicates acidity, a ph of 7 is neutral, and a high ph value indicates alkalinity. The ph value can tell you whether you're dealing with an acid or a base,.. High Vs Low Pka.
From stock.adobe.com
Vecteur Stock Glutamic acid Glu E Non Essential Amino Acid High Vs Low Pka The ph value can tell you whether you're dealing with an acid or a base,. A low ph value indicates acidity, a ph of 7 is neutral, and a high ph value indicates alkalinity. It indicates the strength of an acid and can be used to select buffers. It measures the strength of an acid by how tightly. Pka is. High Vs Low Pka.
From japaneseclass.jp
PKA PKA JapaneseClass.jp High Vs Low Pka The ph value can tell you whether you're dealing with an acid or a base,. It measures the strength of an acid by how tightly. It indicates the strength of an acid and can be used to select buffers. Pka is the negative logarithm of the acid dissociation constant of a solution. Learn how to compare the acidities of different. High Vs Low Pka.
From www.chemistrysteps.com
The pKa in Organic Chemistry Chemistry Steps High Vs Low Pka Learn how to compare the acidities of different compounds using the pka parameter, which measures how tightly a proton is held by. It indicates the strength of an acid and can be used to select buffers. Pka is the negative logarithm of the acid dissociation constant of a solution. Learn about the quantitative measure of acid strength in solution, its. High Vs Low Pka.
From www.askdifference.com
pKa vs. pH — What’s the Difference? High Vs Low Pka It measures the strength of an acid by how tightly. Pka is the negative logarithm of the acid dissociation constant of a solution. Learn how to quantify the acid strength of organic compounds using pka values and periodic trends. Learn how to compare the acidities of different compounds using the pka parameter, which measures how tightly a proton is held. High Vs Low Pka.
From www.masterorganicchemistry.com
How To Use a pKa Table High Vs Low Pka It indicates the strength of an acid and can be used to select buffers. Learn how to calculate the acid ionization constant (ka) and the acid dissociation constant (pka) for weak acids and their. Learn how to calculate pka and ph and. The ph value can tell you whether you're dealing with an acid or a base,. Pka is the. High Vs Low Pka.
From www.masterorganicchemistry.com
How To Use a pKa Table High Vs Low Pka Learn how to calculate the acid ionization constant (ka) and the acid dissociation constant (pka) for weak acids and their. Pka is the negative logarithm of the acid dissociation constant of a solution. It measures the strength of an acid by how tightly. It indicates the strength of an acid and can be used to select buffers. Pka is the. High Vs Low Pka.