Alkane Vs Alkene Vs Alkyne Boiling Point . Compared to alkanes and alkenes, alkynes have a slightly higher boiling pointa. Boiling points of alkenes depends on more molecular mass (chain length). An organic molecule is one in which there is at least one atom of carbon, while a hydrocarbon is. Alkanes, alkenes, and alkynes are all organic hydrocarbons. Boiling points of alkynes are slightly higher than those of their corresponding alkenes due to one extra bond at the carbon site. Alkynes are slightly electronegative in. Alkenes are more reactive than alkynes due to the presence of a double bond, allowing them to undergo addition reactions with other. The more intermolecular mass is added, the higher the. The more intermolecular mass is added, the higher the boiling point. Boiling points of alkenes depends on more molecular mass (chain length).
from www.youtube.com
Alkynes are slightly electronegative in. An organic molecule is one in which there is at least one atom of carbon, while a hydrocarbon is. Alkanes, alkenes, and alkynes are all organic hydrocarbons. The more intermolecular mass is added, the higher the. Boiling points of alkenes depends on more molecular mass (chain length). Boiling points of alkynes are slightly higher than those of their corresponding alkenes due to one extra bond at the carbon site. Compared to alkanes and alkenes, alkynes have a slightly higher boiling pointa. The more intermolecular mass is added, the higher the boiling point. Boiling points of alkenes depends on more molecular mass (chain length). Alkenes are more reactive than alkynes due to the presence of a double bond, allowing them to undergo addition reactions with other.
Comparing alkane with alkene YouTube
Alkane Vs Alkene Vs Alkyne Boiling Point Boiling points of alkenes depends on more molecular mass (chain length). Alkanes, alkenes, and alkynes are all organic hydrocarbons. Boiling points of alkynes are slightly higher than those of their corresponding alkenes due to one extra bond at the carbon site. The more intermolecular mass is added, the higher the boiling point. Alkenes are more reactive than alkynes due to the presence of a double bond, allowing them to undergo addition reactions with other. Boiling points of alkenes depends on more molecular mass (chain length). The more intermolecular mass is added, the higher the. Alkynes are slightly electronegative in. Boiling points of alkenes depends on more molecular mass (chain length). Compared to alkanes and alkenes, alkynes have a slightly higher boiling pointa. An organic molecule is one in which there is at least one atom of carbon, while a hydrocarbon is.
From selfstudypoint.in
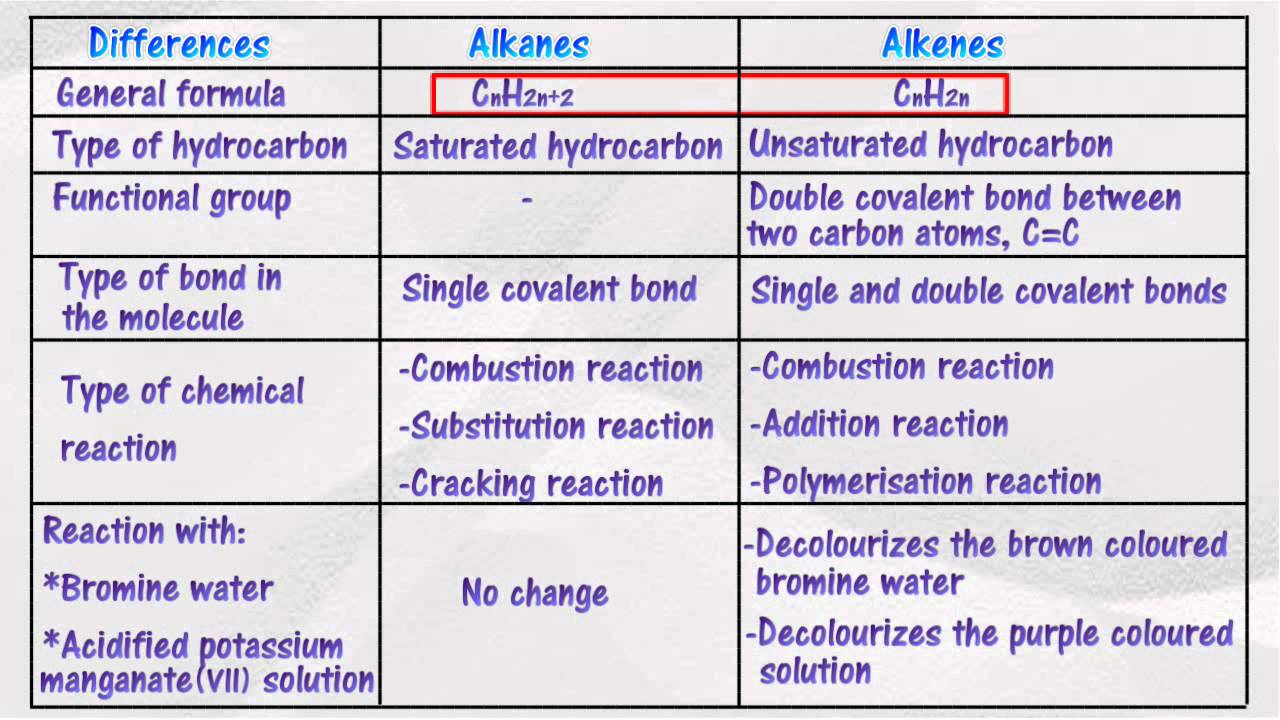
Comparative Study of Alkanes, Alkenes, Alkynes Alkane Vs Alkene Vs Alkyne Boiling Point An organic molecule is one in which there is at least one atom of carbon, while a hydrocarbon is. Alkynes are slightly electronegative in. Boiling points of alkenes depends on more molecular mass (chain length). The more intermolecular mass is added, the higher the boiling point. Compared to alkanes and alkenes, alkynes have a slightly higher boiling pointa. Boiling points. Alkane Vs Alkene Vs Alkyne Boiling Point.
From wou.edu
CH105 Chapter 8 Alkenes, Alkynes and Aromatic Compounds Chemistry Alkane Vs Alkene Vs Alkyne Boiling Point Alkenes are more reactive than alkynes due to the presence of a double bond, allowing them to undergo addition reactions with other. Boiling points of alkenes depends on more molecular mass (chain length). An organic molecule is one in which there is at least one atom of carbon, while a hydrocarbon is. Alkanes, alkenes, and alkynes are all organic hydrocarbons.. Alkane Vs Alkene Vs Alkyne Boiling Point.
From www.studypool.com
SOLUTION Alkanes alkenes alkynes and aromatic compounds Studypool Alkane Vs Alkene Vs Alkyne Boiling Point The more intermolecular mass is added, the higher the boiling point. Alkynes are slightly electronegative in. The more intermolecular mass is added, the higher the. Compared to alkanes and alkenes, alkynes have a slightly higher boiling pointa. An organic molecule is one in which there is at least one atom of carbon, while a hydrocarbon is. Alkenes are more reactive. Alkane Vs Alkene Vs Alkyne Boiling Point.
From www.coursehero.com
[Solved] B. Distinguish between 1. Alkane and alkene 2. Alkene and Alkane Vs Alkene Vs Alkyne Boiling Point Alkanes, alkenes, and alkynes are all organic hydrocarbons. Boiling points of alkenes depends on more molecular mass (chain length). Alkenes are more reactive than alkynes due to the presence of a double bond, allowing them to undergo addition reactions with other. Boiling points of alkenes depends on more molecular mass (chain length). Boiling points of alkynes are slightly higher than. Alkane Vs Alkene Vs Alkyne Boiling Point.
From owlcation.com
The Chemistry of Alkenes Structure, Naming, Uses & Reactions Owlcation Alkane Vs Alkene Vs Alkyne Boiling Point Boiling points of alkenes depends on more molecular mass (chain length). The more intermolecular mass is added, the higher the. Alkenes are more reactive than alkynes due to the presence of a double bond, allowing them to undergo addition reactions with other. An organic molecule is one in which there is at least one atom of carbon, while a hydrocarbon. Alkane Vs Alkene Vs Alkyne Boiling Point.
From www.youtube.com
Comparing alkane with alkene YouTube Alkane Vs Alkene Vs Alkyne Boiling Point The more intermolecular mass is added, the higher the. Alkanes, alkenes, and alkynes are all organic hydrocarbons. Boiling points of alkenes depends on more molecular mass (chain length). Boiling points of alkenes depends on more molecular mass (chain length). Compared to alkanes and alkenes, alkynes have a slightly higher boiling pointa. The more intermolecular mass is added, the higher the. Alkane Vs Alkene Vs Alkyne Boiling Point.
From byjus.com
Explain the order of boiling pt between alkane alkene alkyne in detail. Alkane Vs Alkene Vs Alkyne Boiling Point An organic molecule is one in which there is at least one atom of carbon, while a hydrocarbon is. The more intermolecular mass is added, the higher the. Boiling points of alkenes depends on more molecular mass (chain length). Boiling points of alkynes are slightly higher than those of their corresponding alkenes due to one extra bond at the carbon. Alkane Vs Alkene Vs Alkyne Boiling Point.
From brainly.com
Question 1 Alkanes, Alkenes, and Alkynes Complete the table by Alkane Vs Alkene Vs Alkyne Boiling Point Boiling points of alkynes are slightly higher than those of their corresponding alkenes due to one extra bond at the carbon site. Alkanes, alkenes, and alkynes are all organic hydrocarbons. Alkynes are slightly electronegative in. Alkenes are more reactive than alkynes due to the presence of a double bond, allowing them to undergo addition reactions with other. Compared to alkanes. Alkane Vs Alkene Vs Alkyne Boiling Point.
From chemistryclinic.co.uk
Alkanes and Alkenes Chemistry Alkane Vs Alkene Vs Alkyne Boiling Point Alkynes are slightly electronegative in. The more intermolecular mass is added, the higher the. Alkanes, alkenes, and alkynes are all organic hydrocarbons. Boiling points of alkenes depends on more molecular mass (chain length). Boiling points of alkenes depends on more molecular mass (chain length). An organic molecule is one in which there is at least one atom of carbon, while. Alkane Vs Alkene Vs Alkyne Boiling Point.
From www.youtube.com
Alkanes, Alkenes, Alkynes (4/11) Organic Chemistry NCEA Level 2 Alkane Vs Alkene Vs Alkyne Boiling Point Compared to alkanes and alkenes, alkynes have a slightly higher boiling pointa. Alkenes are more reactive than alkynes due to the presence of a double bond, allowing them to undergo addition reactions with other. Boiling points of alkynes are slightly higher than those of their corresponding alkenes due to one extra bond at the carbon site. The more intermolecular mass. Alkane Vs Alkene Vs Alkyne Boiling Point.
From slidetodoc.com
Content Alkane Structure and physical properties boiling point Alkane Vs Alkene Vs Alkyne Boiling Point Boiling points of alkenes depends on more molecular mass (chain length). Alkynes are slightly electronegative in. Alkanes, alkenes, and alkynes are all organic hydrocarbons. Boiling points of alkynes are slightly higher than those of their corresponding alkenes due to one extra bond at the carbon site. Compared to alkanes and alkenes, alkynes have a slightly higher boiling pointa. Alkenes are. Alkane Vs Alkene Vs Alkyne Boiling Point.
From chem.libretexts.org
2.1 Alkenes Structures and Names Chemistry LibreTexts Alkane Vs Alkene Vs Alkyne Boiling Point The more intermolecular mass is added, the higher the boiling point. Alkynes are slightly electronegative in. Compared to alkanes and alkenes, alkynes have a slightly higher boiling pointa. Alkenes are more reactive than alkynes due to the presence of a double bond, allowing them to undergo addition reactions with other. Boiling points of alkenes depends on more molecular mass (chain. Alkane Vs Alkene Vs Alkyne Boiling Point.
From mungfali.com
Naming Alkenes Examples Alkane Vs Alkene Vs Alkyne Boiling Point Compared to alkanes and alkenes, alkynes have a slightly higher boiling pointa. The more intermolecular mass is added, the higher the boiling point. Alkenes are more reactive than alkynes due to the presence of a double bond, allowing them to undergo addition reactions with other. An organic molecule is one in which there is at least one atom of carbon,. Alkane Vs Alkene Vs Alkyne Boiling Point.
From simp-link.com
C9h18 alkane alkene or alkyne Alkane Vs Alkene Vs Alkyne Boiling Point The more intermolecular mass is added, the higher the boiling point. Alkenes are more reactive than alkynes due to the presence of a double bond, allowing them to undergo addition reactions with other. An organic molecule is one in which there is at least one atom of carbon, while a hydrocarbon is. Boiling points of alkynes are slightly higher than. Alkane Vs Alkene Vs Alkyne Boiling Point.
From www.vrogue.co
Alkanes Vs Alkenes Vs Alkynes Chemistry Lessons Organ vrogue.co Alkane Vs Alkene Vs Alkyne Boiling Point Alkenes are more reactive than alkynes due to the presence of a double bond, allowing them to undergo addition reactions with other. Alkynes are slightly electronegative in. Compared to alkanes and alkenes, alkynes have a slightly higher boiling pointa. The more intermolecular mass is added, the higher the boiling point. Boiling points of alkenes depends on more molecular mass (chain. Alkane Vs Alkene Vs Alkyne Boiling Point.
From simp-link.com
C9h18 alkane alkene or alkyne Alkane Vs Alkene Vs Alkyne Boiling Point Compared to alkanes and alkenes, alkynes have a slightly higher boiling pointa. The more intermolecular mass is added, the higher the. Alkenes are more reactive than alkynes due to the presence of a double bond, allowing them to undergo addition reactions with other. An organic molecule is one in which there is at least one atom of carbon, while a. Alkane Vs Alkene Vs Alkyne Boiling Point.
From pediaa.com
Difference Between Alkanes and Alkenes Definition, Nomenclature Alkane Vs Alkene Vs Alkyne Boiling Point The more intermolecular mass is added, the higher the. Alkanes, alkenes, and alkynes are all organic hydrocarbons. An organic molecule is one in which there is at least one atom of carbon, while a hydrocarbon is. Alkenes are more reactive than alkynes due to the presence of a double bond, allowing them to undergo addition reactions with other. Boiling points. Alkane Vs Alkene Vs Alkyne Boiling Point.
From www.youtube.com
Differences Between Alkane, Alkene, and Alkyne. YouTube Alkane Vs Alkene Vs Alkyne Boiling Point An organic molecule is one in which there is at least one atom of carbon, while a hydrocarbon is. Compared to alkanes and alkenes, alkynes have a slightly higher boiling pointa. Boiling points of alkynes are slightly higher than those of their corresponding alkenes due to one extra bond at the carbon site. Boiling points of alkenes depends on more. Alkane Vs Alkene Vs Alkyne Boiling Point.
From www.slideserve.com
PPT Alkanes vs. Alkenes PowerPoint Presentation, free download ID Alkane Vs Alkene Vs Alkyne Boiling Point Boiling points of alkenes depends on more molecular mass (chain length). The more intermolecular mass is added, the higher the. Compared to alkanes and alkenes, alkynes have a slightly higher boiling pointa. Alkanes, alkenes, and alkynes are all organic hydrocarbons. Boiling points of alkenes depends on more molecular mass (chain length). An organic molecule is one in which there is. Alkane Vs Alkene Vs Alkyne Boiling Point.
From slideplayer.com
Properties and Synthesis ppt download Alkane Vs Alkene Vs Alkyne Boiling Point Boiling points of alkenes depends on more molecular mass (chain length). Alkanes, alkenes, and alkynes are all organic hydrocarbons. The more intermolecular mass is added, the higher the. An organic molecule is one in which there is at least one atom of carbon, while a hydrocarbon is. The more intermolecular mass is added, the higher the boiling point. Alkenes are. Alkane Vs Alkene Vs Alkyne Boiling Point.
From www.differencebetween.com
Difference Between Alkenes and Alkynes Compare the Difference Between Alkane Vs Alkene Vs Alkyne Boiling Point Boiling points of alkynes are slightly higher than those of their corresponding alkenes due to one extra bond at the carbon site. Boiling points of alkenes depends on more molecular mass (chain length). Alkenes are more reactive than alkynes due to the presence of a double bond, allowing them to undergo addition reactions with other. Alkanes, alkenes, and alkynes are. Alkane Vs Alkene Vs Alkyne Boiling Point.
From www.doubtnut.com
Alkane and Alkene Alkane Vs Alkene Vs Alkyne Boiling Point The more intermolecular mass is added, the higher the boiling point. An organic molecule is one in which there is at least one atom of carbon, while a hydrocarbon is. The more intermolecular mass is added, the higher the. Alkanes, alkenes, and alkynes are all organic hydrocarbons. Boiling points of alkynes are slightly higher than those of their corresponding alkenes. Alkane Vs Alkene Vs Alkyne Boiling Point.
From chemistnotes.com
Alkenes formula, structure, nomenclature, properties, and uses Alkane Vs Alkene Vs Alkyne Boiling Point Boiling points of alkynes are slightly higher than those of their corresponding alkenes due to one extra bond at the carbon site. Alkynes are slightly electronegative in. An organic molecule is one in which there is at least one atom of carbon, while a hydrocarbon is. The more intermolecular mass is added, the higher the boiling point. Alkanes, alkenes, and. Alkane Vs Alkene Vs Alkyne Boiling Point.
From elearning.reb.rw
Course Chemistry, Topic Unit 10 Properties of Organic Compounds and Alkane Vs Alkene Vs Alkyne Boiling Point Compared to alkanes and alkenes, alkynes have a slightly higher boiling pointa. Alkanes, alkenes, and alkynes are all organic hydrocarbons. The more intermolecular mass is added, the higher the boiling point. Alkenes are more reactive than alkynes due to the presence of a double bond, allowing them to undergo addition reactions with other. Alkynes are slightly electronegative in. Boiling points. Alkane Vs Alkene Vs Alkyne Boiling Point.
From www.youtube.com
Alkanes, Alkenes, and Alkynes General molecular formula Chemistry Alkane Vs Alkene Vs Alkyne Boiling Point Alkenes are more reactive than alkynes due to the presence of a double bond, allowing them to undergo addition reactions with other. Alkynes are slightly electronegative in. Boiling points of alkynes are slightly higher than those of their corresponding alkenes due to one extra bond at the carbon site. Alkanes, alkenes, and alkynes are all organic hydrocarbons. An organic molecule. Alkane Vs Alkene Vs Alkyne Boiling Point.
From www.engineeringtoolbox.com
Hydrocarbons, Alcohols and Acids Boiling points Alkane Vs Alkene Vs Alkyne Boiling Point The more intermolecular mass is added, the higher the. Compared to alkanes and alkenes, alkynes have a slightly higher boiling pointa. An organic molecule is one in which there is at least one atom of carbon, while a hydrocarbon is. The more intermolecular mass is added, the higher the boiling point. Alkenes are more reactive than alkynes due to the. Alkane Vs Alkene Vs Alkyne Boiling Point.
From www.slideshare.net
Alkane,alkene,alkyne Alkane Vs Alkene Vs Alkyne Boiling Point Alkynes are slightly electronegative in. Boiling points of alkenes depends on more molecular mass (chain length). Alkanes, alkenes, and alkynes are all organic hydrocarbons. The more intermolecular mass is added, the higher the boiling point. Compared to alkanes and alkenes, alkynes have a slightly higher boiling pointa. An organic molecule is one in which there is at least one atom. Alkane Vs Alkene Vs Alkyne Boiling Point.
From www.shutterstock.com
Alkane Alkene Alkyne Functional Groups Organic Stock Illustration Alkane Vs Alkene Vs Alkyne Boiling Point Boiling points of alkenes depends on more molecular mass (chain length). Alkenes are more reactive than alkynes due to the presence of a double bond, allowing them to undergo addition reactions with other. Boiling points of alkynes are slightly higher than those of their corresponding alkenes due to one extra bond at the carbon site. An organic molecule is one. Alkane Vs Alkene Vs Alkyne Boiling Point.
From www.pinterest.com.au
Naming alkenes follows the same rules we discussed earlier for the Alkane Vs Alkene Vs Alkyne Boiling Point Alkanes, alkenes, and alkynes are all organic hydrocarbons. Alkynes are slightly electronegative in. The more intermolecular mass is added, the higher the. Boiling points of alkynes are slightly higher than those of their corresponding alkenes due to one extra bond at the carbon site. Boiling points of alkenes depends on more molecular mass (chain length). An organic molecule is one. Alkane Vs Alkene Vs Alkyne Boiling Point.
From simp-link.com
C9h18 alkane alkene or alkyne Alkane Vs Alkene Vs Alkyne Boiling Point The more intermolecular mass is added, the higher the. An organic molecule is one in which there is at least one atom of carbon, while a hydrocarbon is. Boiling points of alkenes depends on more molecular mass (chain length). Compared to alkanes and alkenes, alkynes have a slightly higher boiling pointa. Boiling points of alkenes depends on more molecular mass. Alkane Vs Alkene Vs Alkyne Boiling Point.
From www.youtube.com
The correct boiling point order for correspondins hydrocarbons is (A Alkane Vs Alkene Vs Alkyne Boiling Point The more intermolecular mass is added, the higher the boiling point. Boiling points of alkenes depends on more molecular mass (chain length). An organic molecule is one in which there is at least one atom of carbon, while a hydrocarbon is. The more intermolecular mass is added, the higher the. Alkanes, alkenes, and alkynes are all organic hydrocarbons. Alkenes are. Alkane Vs Alkene Vs Alkyne Boiling Point.
From chemistryjee.blogspot.com
chemistry world Alkanes Physical Properties Alkane Vs Alkene Vs Alkyne Boiling Point Compared to alkanes and alkenes, alkynes have a slightly higher boiling pointa. Alkynes are slightly electronegative in. Alkanes, alkenes, and alkynes are all organic hydrocarbons. The more intermolecular mass is added, the higher the. The more intermolecular mass is added, the higher the boiling point. Boiling points of alkenes depends on more molecular mass (chain length). Alkenes are more reactive. Alkane Vs Alkene Vs Alkyne Boiling Point.
From www.numerade.com
SOLVED Q3. What do you think will be the boiling point of the next Alkane Vs Alkene Vs Alkyne Boiling Point Compared to alkanes and alkenes, alkynes have a slightly higher boiling pointa. Alkenes are more reactive than alkynes due to the presence of a double bond, allowing them to undergo addition reactions with other. The more intermolecular mass is added, the higher the. Boiling points of alkenes depends on more molecular mass (chain length). Alkynes are slightly electronegative in. An. Alkane Vs Alkene Vs Alkyne Boiling Point.
From www.youtube.com
Comparing the Boiling Point of Alkanes Organic Chemistry YouTube Alkane Vs Alkene Vs Alkyne Boiling Point Boiling points of alkenes depends on more molecular mass (chain length). The more intermolecular mass is added, the higher the. The more intermolecular mass is added, the higher the boiling point. Alkynes are slightly electronegative in. Boiling points of alkenes depends on more molecular mass (chain length). Alkenes are more reactive than alkynes due to the presence of a double. Alkane Vs Alkene Vs Alkyne Boiling Point.
From www.wou.edu
CH105 Chapter 7 Alkanes and Halogenated Hydrocarbons Chemistry Alkane Vs Alkene Vs Alkyne Boiling Point Alkynes are slightly electronegative in. Boiling points of alkenes depends on more molecular mass (chain length). Boiling points of alkenes depends on more molecular mass (chain length). Compared to alkanes and alkenes, alkynes have a slightly higher boiling pointa. Alkenes are more reactive than alkynes due to the presence of a double bond, allowing them to undergo addition reactions with. Alkane Vs Alkene Vs Alkyne Boiling Point.