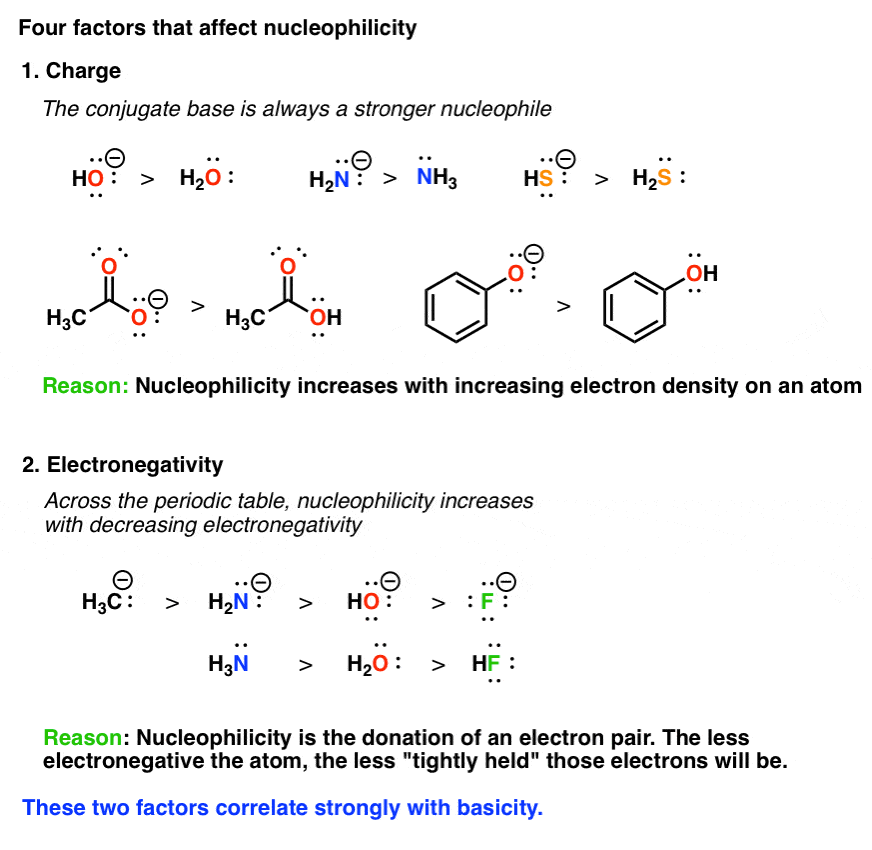
Are Aromatic Compounds Good Nucleophiles . Write the detailed mechanism for a nucleophilic aromatic substitution reaction. The four key conditions a molecule must fulfill if it is to be aromatic, with lots of. Four factors that determine what makes a good nucleophile are its charge, electronegativity, the solvent, and the steric. Identify the conditions necessary for an aryl halide to undergo nucleophilic aromatic substitution, and give an example of such a reaction. Normally, aromatic rings are considered electron rich and are good nucleophiles in the classic electrophilic. In this post we go through the rules for aromaticity:
from www.masterorganicchemistry.com
In this post we go through the rules for aromaticity: Identify the conditions necessary for an aryl halide to undergo nucleophilic aromatic substitution, and give an example of such a reaction. Write the detailed mechanism for a nucleophilic aromatic substitution reaction. The four key conditions a molecule must fulfill if it is to be aromatic, with lots of. Four factors that determine what makes a good nucleophile are its charge, electronegativity, the solvent, and the steric. Normally, aromatic rings are considered electron rich and are good nucleophiles in the classic electrophilic.
What Makes A Good Nucleophile? — Master Organic Chemistry
Are Aromatic Compounds Good Nucleophiles Four factors that determine what makes a good nucleophile are its charge, electronegativity, the solvent, and the steric. Write the detailed mechanism for a nucleophilic aromatic substitution reaction. Identify the conditions necessary for an aryl halide to undergo nucleophilic aromatic substitution, and give an example of such a reaction. Normally, aromatic rings are considered electron rich and are good nucleophiles in the classic electrophilic. Four factors that determine what makes a good nucleophile are its charge, electronegativity, the solvent, and the steric. The four key conditions a molecule must fulfill if it is to be aromatic, with lots of. In this post we go through the rules for aromaticity:
From www.thesciencehive.co.uk
Aromatic compounds — the science hive Are Aromatic Compounds Good Nucleophiles Normally, aromatic rings are considered electron rich and are good nucleophiles in the classic electrophilic. Identify the conditions necessary for an aryl halide to undergo nucleophilic aromatic substitution, and give an example of such a reaction. The four key conditions a molecule must fulfill if it is to be aromatic, with lots of. Four factors that determine what makes a. Are Aromatic Compounds Good Nucleophiles.
From www.organicchemistrytutor.com
Nucleophiles and Electrophiles — Organic Chemistry Tutor Are Aromatic Compounds Good Nucleophiles Four factors that determine what makes a good nucleophile are its charge, electronegativity, the solvent, and the steric. The four key conditions a molecule must fulfill if it is to be aromatic, with lots of. In this post we go through the rules for aromaticity: Normally, aromatic rings are considered electron rich and are good nucleophiles in the classic electrophilic.. Are Aromatic Compounds Good Nucleophiles.
From www.mdpi.com
Molecules Free FullText How Do Aromatic Nitro Compounds React with Are Aromatic Compounds Good Nucleophiles Identify the conditions necessary for an aryl halide to undergo nucleophilic aromatic substitution, and give an example of such a reaction. Four factors that determine what makes a good nucleophile are its charge, electronegativity, the solvent, and the steric. Normally, aromatic rings are considered electron rich and are good nucleophiles in the classic electrophilic. The four key conditions a molecule. Are Aromatic Compounds Good Nucleophiles.
From www.chemistrysteps.com
Nucleophilic Aromatic Substitution Chemistry Steps Are Aromatic Compounds Good Nucleophiles Write the detailed mechanism for a nucleophilic aromatic substitution reaction. The four key conditions a molecule must fulfill if it is to be aromatic, with lots of. In this post we go through the rules for aromaticity: Normally, aromatic rings are considered electron rich and are good nucleophiles in the classic electrophilic. Four factors that determine what makes a good. Are Aromatic Compounds Good Nucleophiles.
From www.yumpu.com
A.2 Nucleophilic Aromatic Substitution Are Aromatic Compounds Good Nucleophiles In this post we go through the rules for aromaticity: The four key conditions a molecule must fulfill if it is to be aromatic, with lots of. Normally, aromatic rings are considered electron rich and are good nucleophiles in the classic electrophilic. Four factors that determine what makes a good nucleophile are its charge, electronegativity, the solvent, and the steric.. Are Aromatic Compounds Good Nucleophiles.
From www.chemistrylearner.com
Nucleophilic Aromatic Substitution Definition & Mechanism Are Aromatic Compounds Good Nucleophiles In this post we go through the rules for aromaticity: Normally, aromatic rings are considered electron rich and are good nucleophiles in the classic electrophilic. Four factors that determine what makes a good nucleophile are its charge, electronegativity, the solvent, and the steric. Identify the conditions necessary for an aryl halide to undergo nucleophilic aromatic substitution, and give an example. Are Aromatic Compounds Good Nucleophiles.
From byjus.com
Aromatic Compounds Definition, Example, Properties & Nomenclature Are Aromatic Compounds Good Nucleophiles Write the detailed mechanism for a nucleophilic aromatic substitution reaction. Normally, aromatic rings are considered electron rich and are good nucleophiles in the classic electrophilic. The four key conditions a molecule must fulfill if it is to be aromatic, with lots of. In this post we go through the rules for aromaticity: Four factors that determine what makes a good. Are Aromatic Compounds Good Nucleophiles.
From www.chemistrysteps.com
Nucleophilic Aromatic Substitution Chemistry Steps Are Aromatic Compounds Good Nucleophiles The four key conditions a molecule must fulfill if it is to be aromatic, with lots of. Identify the conditions necessary for an aryl halide to undergo nucleophilic aromatic substitution, and give an example of such a reaction. In this post we go through the rules for aromaticity: Four factors that determine what makes a good nucleophile are its charge,. Are Aromatic Compounds Good Nucleophiles.
From www.masterorganicchemistry.com
Nucleophilic Aromatic Substitution Introduction and Mechanism Are Aromatic Compounds Good Nucleophiles Normally, aromatic rings are considered electron rich and are good nucleophiles in the classic electrophilic. In this post we go through the rules for aromaticity: The four key conditions a molecule must fulfill if it is to be aromatic, with lots of. Identify the conditions necessary for an aryl halide to undergo nucleophilic aromatic substitution, and give an example of. Are Aromatic Compounds Good Nucleophiles.
From www.masterorganicchemistry.com
Nucleophilic Aromatic Substitution The Benzyne Mechanism Are Aromatic Compounds Good Nucleophiles In this post we go through the rules for aromaticity: Normally, aromatic rings are considered electron rich and are good nucleophiles in the classic electrophilic. Four factors that determine what makes a good nucleophile are its charge, electronegativity, the solvent, and the steric. Write the detailed mechanism for a nucleophilic aromatic substitution reaction. Identify the conditions necessary for an aryl. Are Aromatic Compounds Good Nucleophiles.
From www.masterorganicchemistry.com
What Makes A Good Nucleophile? Master Organic Chemistry Are Aromatic Compounds Good Nucleophiles The four key conditions a molecule must fulfill if it is to be aromatic, with lots of. Identify the conditions necessary for an aryl halide to undergo nucleophilic aromatic substitution, and give an example of such a reaction. Normally, aromatic rings are considered electron rich and are good nucleophiles in the classic electrophilic. Four factors that determine what makes a. Are Aromatic Compounds Good Nucleophiles.
From www.semanticscholar.org
Table 1 from How Do Aromatic Nitro Compounds React with Nucleophiles Are Aromatic Compounds Good Nucleophiles The four key conditions a molecule must fulfill if it is to be aromatic, with lots of. Four factors that determine what makes a good nucleophile are its charge, electronegativity, the solvent, and the steric. Identify the conditions necessary for an aryl halide to undergo nucleophilic aromatic substitution, and give an example of such a reaction. In this post we. Are Aromatic Compounds Good Nucleophiles.
From www.masterorganicchemistry.com
Nucleophilic Aromatic Substitution Introduction and Mechanism Are Aromatic Compounds Good Nucleophiles The four key conditions a molecule must fulfill if it is to be aromatic, with lots of. Normally, aromatic rings are considered electron rich and are good nucleophiles in the classic electrophilic. In this post we go through the rules for aromaticity: Four factors that determine what makes a good nucleophile are its charge, electronegativity, the solvent, and the steric.. Are Aromatic Compounds Good Nucleophiles.
From www.masterorganicchemistry.com
Nucleophilic Aromatic Substitution Introduction and Mechanism Are Aromatic Compounds Good Nucleophiles Normally, aromatic rings are considered electron rich and are good nucleophiles in the classic electrophilic. In this post we go through the rules for aromaticity: Write the detailed mechanism for a nucleophilic aromatic substitution reaction. Four factors that determine what makes a good nucleophile are its charge, electronegativity, the solvent, and the steric. The four key conditions a molecule must. Are Aromatic Compounds Good Nucleophiles.
From www.masterorganicchemistry.com
What Makes A Good Nucleophile? Master Organic Chemistry Are Aromatic Compounds Good Nucleophiles Identify the conditions necessary for an aryl halide to undergo nucleophilic aromatic substitution, and give an example of such a reaction. Normally, aromatic rings are considered electron rich and are good nucleophiles in the classic electrophilic. In this post we go through the rules for aromaticity: The four key conditions a molecule must fulfill if it is to be aromatic,. Are Aromatic Compounds Good Nucleophiles.
From www.chemistrysteps.com
Nucleophilic Aromatic Substitution Chemistry Steps Are Aromatic Compounds Good Nucleophiles Identify the conditions necessary for an aryl halide to undergo nucleophilic aromatic substitution, and give an example of such a reaction. The four key conditions a molecule must fulfill if it is to be aromatic, with lots of. In this post we go through the rules for aromaticity: Normally, aromatic rings are considered electron rich and are good nucleophiles in. Are Aromatic Compounds Good Nucleophiles.
From www.masterorganicchemistry.com
Deciding SN1/SN2/E1/E2 (2) The Nucleophile/Base Are Aromatic Compounds Good Nucleophiles In this post we go through the rules for aromaticity: The four key conditions a molecule must fulfill if it is to be aromatic, with lots of. Identify the conditions necessary for an aryl halide to undergo nucleophilic aromatic substitution, and give an example of such a reaction. Write the detailed mechanism for a nucleophilic aromatic substitution reaction. Normally, aromatic. Are Aromatic Compounds Good Nucleophiles.
From www.chemistrysteps.com
Nucleophilic Aromatic Substitution Chemistry Steps Are Aromatic Compounds Good Nucleophiles Four factors that determine what makes a good nucleophile are its charge, electronegativity, the solvent, and the steric. Write the detailed mechanism for a nucleophilic aromatic substitution reaction. Identify the conditions necessary for an aryl halide to undergo nucleophilic aromatic substitution, and give an example of such a reaction. The four key conditions a molecule must fulfill if it is. Are Aromatic Compounds Good Nucleophiles.
From www.mdpi.com
Molecules Free FullText Analogy of the Reactions of Aromatic and Are Aromatic Compounds Good Nucleophiles Normally, aromatic rings are considered electron rich and are good nucleophiles in the classic electrophilic. Write the detailed mechanism for a nucleophilic aromatic substitution reaction. Four factors that determine what makes a good nucleophile are its charge, electronegativity, the solvent, and the steric. In this post we go through the rules for aromaticity: The four key conditions a molecule must. Are Aromatic Compounds Good Nucleophiles.
From www.bartleby.com
Aromatic Compounds bartleby Are Aromatic Compounds Good Nucleophiles Four factors that determine what makes a good nucleophile are its charge, electronegativity, the solvent, and the steric. Normally, aromatic rings are considered electron rich and are good nucleophiles in the classic electrophilic. Write the detailed mechanism for a nucleophilic aromatic substitution reaction. In this post we go through the rules for aromaticity: Identify the conditions necessary for an aryl. Are Aromatic Compounds Good Nucleophiles.
From www.organicchemistrytutor.com
Reactions of Aromatic Compounds — Organic Chemistry Tutor Are Aromatic Compounds Good Nucleophiles The four key conditions a molecule must fulfill if it is to be aromatic, with lots of. Write the detailed mechanism for a nucleophilic aromatic substitution reaction. Normally, aromatic rings are considered electron rich and are good nucleophiles in the classic electrophilic. Identify the conditions necessary for an aryl halide to undergo nucleophilic aromatic substitution, and give an example of. Are Aromatic Compounds Good Nucleophiles.
From www.masterorganicchemistry.com
The three types of nucleophiles you meet in organic chemistry — Master Are Aromatic Compounds Good Nucleophiles In this post we go through the rules for aromaticity: Four factors that determine what makes a good nucleophile are its charge, electronegativity, the solvent, and the steric. Normally, aromatic rings are considered electron rich and are good nucleophiles in the classic electrophilic. The four key conditions a molecule must fulfill if it is to be aromatic, with lots of.. Are Aromatic Compounds Good Nucleophiles.
From www.masterorganicchemistry.com
Rules for Aromaticity The 4 Key Factors Master Organic Chemistry Are Aromatic Compounds Good Nucleophiles Write the detailed mechanism for a nucleophilic aromatic substitution reaction. Normally, aromatic rings are considered electron rich and are good nucleophiles in the classic electrophilic. The four key conditions a molecule must fulfill if it is to be aromatic, with lots of. Four factors that determine what makes a good nucleophile are its charge, electronegativity, the solvent, and the steric.. Are Aromatic Compounds Good Nucleophiles.
From collegedunia.com
Aromatic Compounds Huckel’s Rule, Examples & Properties Are Aromatic Compounds Good Nucleophiles Write the detailed mechanism for a nucleophilic aromatic substitution reaction. Four factors that determine what makes a good nucleophile are its charge, electronegativity, the solvent, and the steric. Normally, aromatic rings are considered electron rich and are good nucleophiles in the classic electrophilic. Identify the conditions necessary for an aryl halide to undergo nucleophilic aromatic substitution, and give an example. Are Aromatic Compounds Good Nucleophiles.
From www.mdpi.com
Molecules Free FullText How Do Aromatic Nitro Compounds React with Are Aromatic Compounds Good Nucleophiles Normally, aromatic rings are considered electron rich and are good nucleophiles in the classic electrophilic. Identify the conditions necessary for an aryl halide to undergo nucleophilic aromatic substitution, and give an example of such a reaction. In this post we go through the rules for aromaticity: The four key conditions a molecule must fulfill if it is to be aromatic,. Are Aromatic Compounds Good Nucleophiles.
From www.chemistrylearner.com
Nucleophilic Aromatic Substitution Definition & Mechanism Are Aromatic Compounds Good Nucleophiles Normally, aromatic rings are considered electron rich and are good nucleophiles in the classic electrophilic. The four key conditions a molecule must fulfill if it is to be aromatic, with lots of. In this post we go through the rules for aromaticity: Four factors that determine what makes a good nucleophile are its charge, electronegativity, the solvent, and the steric.. Are Aromatic Compounds Good Nucleophiles.
From www.masterorganicchemistry.com
The Three Classes of Nucleophiles Master Organic Chemistry Are Aromatic Compounds Good Nucleophiles The four key conditions a molecule must fulfill if it is to be aromatic, with lots of. Four factors that determine what makes a good nucleophile are its charge, electronegativity, the solvent, and the steric. Normally, aromatic rings are considered electron rich and are good nucleophiles in the classic electrophilic. In this post we go through the rules for aromaticity:. Are Aromatic Compounds Good Nucleophiles.
From www.masterorganicchemistry.com
What Makes A Good Nucleophile? — Master Organic Chemistry Are Aromatic Compounds Good Nucleophiles The four key conditions a molecule must fulfill if it is to be aromatic, with lots of. In this post we go through the rules for aromaticity: Four factors that determine what makes a good nucleophile are its charge, electronegativity, the solvent, and the steric. Normally, aromatic rings are considered electron rich and are good nucleophiles in the classic electrophilic.. Are Aromatic Compounds Good Nucleophiles.
From www.masterorganicchemistry.com
Nucleophilic Aromatic Substitution Introduction and Mechanism Are Aromatic Compounds Good Nucleophiles Four factors that determine what makes a good nucleophile are its charge, electronegativity, the solvent, and the steric. Write the detailed mechanism for a nucleophilic aromatic substitution reaction. Identify the conditions necessary for an aryl halide to undergo nucleophilic aromatic substitution, and give an example of such a reaction. Normally, aromatic rings are considered electron rich and are good nucleophiles. Are Aromatic Compounds Good Nucleophiles.
From scienceinfo.com
Aromatic Nucleophilic Substitution Reactions with Types Are Aromatic Compounds Good Nucleophiles Four factors that determine what makes a good nucleophile are its charge, electronegativity, the solvent, and the steric. Identify the conditions necessary for an aryl halide to undergo nucleophilic aromatic substitution, and give an example of such a reaction. In this post we go through the rules for aromaticity: Normally, aromatic rings are considered electron rich and are good nucleophiles. Are Aromatic Compounds Good Nucleophiles.
From www.masterorganicchemistry.com
Nucleophilic Aromatic Substitution Introduction and Mechanism Are Aromatic Compounds Good Nucleophiles Normally, aromatic rings are considered electron rich and are good nucleophiles in the classic electrophilic. Write the detailed mechanism for a nucleophilic aromatic substitution reaction. The four key conditions a molecule must fulfill if it is to be aromatic, with lots of. Identify the conditions necessary for an aryl halide to undergo nucleophilic aromatic substitution, and give an example of. Are Aromatic Compounds Good Nucleophiles.
From www.masterorganicchemistry.com
Nucleophilic Aromatic Substitution The Benzyne Mechanism Are Aromatic Compounds Good Nucleophiles The four key conditions a molecule must fulfill if it is to be aromatic, with lots of. Write the detailed mechanism for a nucleophilic aromatic substitution reaction. In this post we go through the rules for aromaticity: Normally, aromatic rings are considered electron rich and are good nucleophiles in the classic electrophilic. Four factors that determine what makes a good. Are Aromatic Compounds Good Nucleophiles.
From www.chemistrysteps.com
Nucleophilic Aromatic Substitution Chemistry Steps Are Aromatic Compounds Good Nucleophiles The four key conditions a molecule must fulfill if it is to be aromatic, with lots of. Identify the conditions necessary for an aryl halide to undergo nucleophilic aromatic substitution, and give an example of such a reaction. Four factors that determine what makes a good nucleophile are its charge, electronegativity, the solvent, and the steric. Write the detailed mechanism. Are Aromatic Compounds Good Nucleophiles.
From www.chemistrysteps.com
Nucleophilic Aromatic Substitution Chemistry Steps Are Aromatic Compounds Good Nucleophiles The four key conditions a molecule must fulfill if it is to be aromatic, with lots of. Four factors that determine what makes a good nucleophile are its charge, electronegativity, the solvent, and the steric. Write the detailed mechanism for a nucleophilic aromatic substitution reaction. In this post we go through the rules for aromaticity: Normally, aromatic rings are considered. Are Aromatic Compounds Good Nucleophiles.
From www.researchgate.net
Scheme 3. (A,B) Examples of nucleophilic aromatic substitutions on Are Aromatic Compounds Good Nucleophiles Normally, aromatic rings are considered electron rich and are good nucleophiles in the classic electrophilic. Identify the conditions necessary for an aryl halide to undergo nucleophilic aromatic substitution, and give an example of such a reaction. Write the detailed mechanism for a nucleophilic aromatic substitution reaction. Four factors that determine what makes a good nucleophile are its charge, electronegativity, the. Are Aromatic Compounds Good Nucleophiles.