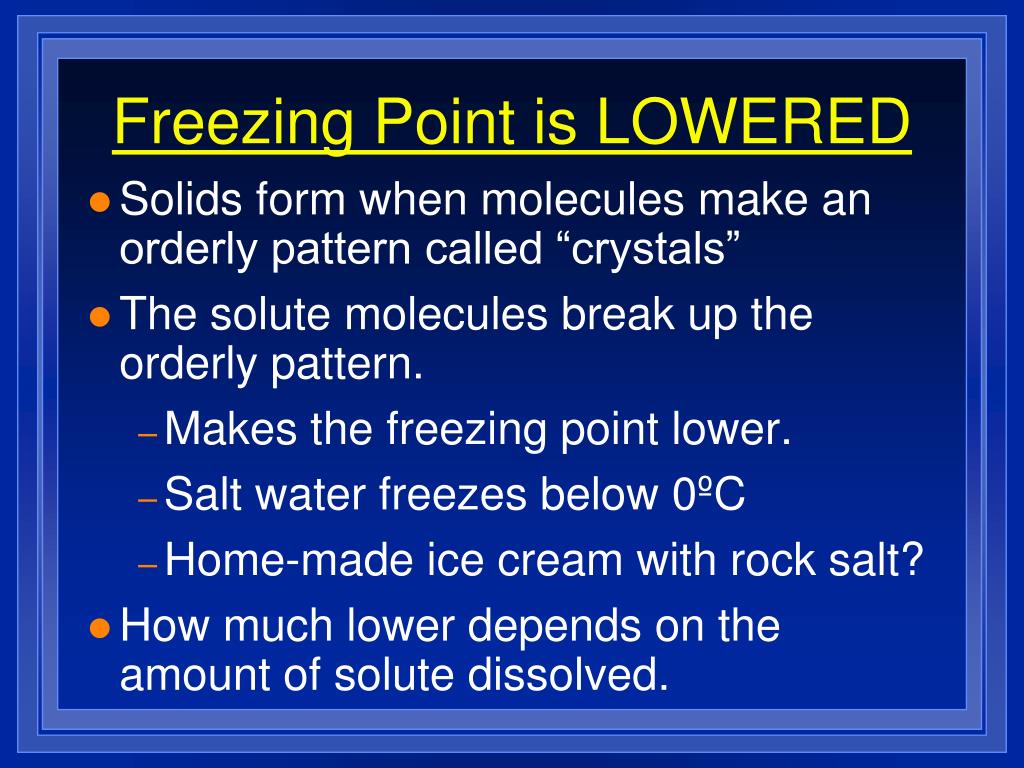
What Does It Mean To Lower The Melting Point . The first is for qualitative identification of the sample, and the second is for quantitative purity. When an impure solid is warmed, microscopic melting first occurs in a pure region by the component with the lower melting point. The melting point is the temperature at which a substance changes from a solid to a liquid. There are two primary uses of melting point analysis data. It says something about the identity of a compound, and something about the purity of a. At the melting point, the change in gibbs free energy of a substance is zero, but the enthalpy and the entropy of the material are. At the melting point, the solid and liquid states both exist and are at equilibrium. The temperature at which solid changes its state to liquid at atmospheric pressure is called. Melting point, temperature at which the solid and liquid forms of a pure substance can exist in equilibrium. The melting point of a compound is useful in two ways: The melting point is usually defined as the point at which materials changes from a solid to a liquid. Impure solids melt at lower temperatures and may also melt over a wider temperature range, known as melting point depression.
from www.slideserve.com
When an impure solid is warmed, microscopic melting first occurs in a pure region by the component with the lower melting point. At the melting point, the solid and liquid states both exist and are at equilibrium. Impure solids melt at lower temperatures and may also melt over a wider temperature range, known as melting point depression. The temperature at which solid changes its state to liquid at atmospheric pressure is called. The melting point is the temperature at which a substance changes from a solid to a liquid. At the melting point, the change in gibbs free energy of a substance is zero, but the enthalpy and the entropy of the material are. The melting point is usually defined as the point at which materials changes from a solid to a liquid. It says something about the identity of a compound, and something about the purity of a. Melting point, temperature at which the solid and liquid forms of a pure substance can exist in equilibrium. The melting point of a compound is useful in two ways:
PPT Chapter 16 “ Solutions ” PowerPoint Presentation, free download
What Does It Mean To Lower The Melting Point The melting point is usually defined as the point at which materials changes from a solid to a liquid. The melting point is usually defined as the point at which materials changes from a solid to a liquid. At the melting point, the solid and liquid states both exist and are at equilibrium. There are two primary uses of melting point analysis data. The first is for qualitative identification of the sample, and the second is for quantitative purity. Impure solids melt at lower temperatures and may also melt over a wider temperature range, known as melting point depression. The temperature at which solid changes its state to liquid at atmospheric pressure is called. It says something about the identity of a compound, and something about the purity of a. The melting point is the temperature at which a substance changes from a solid to a liquid. When an impure solid is warmed, microscopic melting first occurs in a pure region by the component with the lower melting point. The melting point of a compound is useful in two ways: Melting point, temperature at which the solid and liquid forms of a pure substance can exist in equilibrium. At the melting point, the change in gibbs free energy of a substance is zero, but the enthalpy and the entropy of the material are.
From www.slideserve.com
PPT Intermolecular Forces PowerPoint Presentation, free download ID What Does It Mean To Lower The Melting Point At the melting point, the change in gibbs free energy of a substance is zero, but the enthalpy and the entropy of the material are. The melting point is usually defined as the point at which materials changes from a solid to a liquid. There are two primary uses of melting point analysis data. When an impure solid is warmed,. What Does It Mean To Lower The Melting Point.
From www.slideserve.com
PPT Do Now PowerPoint Presentation, free download ID4643426 What Does It Mean To Lower The Melting Point At the melting point, the change in gibbs free energy of a substance is zero, but the enthalpy and the entropy of the material are. The melting point of a compound is useful in two ways: There are two primary uses of melting point analysis data. It says something about the identity of a compound, and something about the purity. What Does It Mean To Lower The Melting Point.
From ksa.mytutorsource.com
Water Freezing Point Definition, Factors Affecting It & Supercooled What Does It Mean To Lower The Melting Point The temperature at which solid changes its state to liquid at atmospheric pressure is called. There are two primary uses of melting point analysis data. When an impure solid is warmed, microscopic melting first occurs in a pure region by the component with the lower melting point. The first is for qualitative identification of the sample, and the second is. What Does It Mean To Lower The Melting Point.
From www.slideserve.com
PPT Melting Point PowerPoint Presentation, free download ID9658296 What Does It Mean To Lower The Melting Point The first is for qualitative identification of the sample, and the second is for quantitative purity. At the melting point, the solid and liquid states both exist and are at equilibrium. Melting point, temperature at which the solid and liquid forms of a pure substance can exist in equilibrium. There are two primary uses of melting point analysis data. The. What Does It Mean To Lower The Melting Point.
From stock.adobe.com
Boiling and Evaporation, Freezing and Melting Points of Water. Stock What Does It Mean To Lower The Melting Point At the melting point, the solid and liquid states both exist and are at equilibrium. At the melting point, the change in gibbs free energy of a substance is zero, but the enthalpy and the entropy of the material are. The first is for qualitative identification of the sample, and the second is for quantitative purity. The melting point of. What Does It Mean To Lower The Melting Point.
From sciencenotes.org
Melting Point Definition and List What Does It Mean To Lower The Melting Point The melting point is usually defined as the point at which materials changes from a solid to a liquid. At the melting point, the change in gibbs free energy of a substance is zero, but the enthalpy and the entropy of the material are. Impure solids melt at lower temperatures and may also melt over a wider temperature range, known. What Does It Mean To Lower The Melting Point.
From www.pinterest.com
Melting Point Easy Science Chemistry education, Learn physics What Does It Mean To Lower The Melting Point The first is for qualitative identification of the sample, and the second is for quantitative purity. At the melting point, the change in gibbs free energy of a substance is zero, but the enthalpy and the entropy of the material are. Melting point, temperature at which the solid and liquid forms of a pure substance can exist in equilibrium. The. What Does It Mean To Lower The Melting Point.
From owlcation.com
What Are the Freezing, Melting, and Boiling Points of Solids, Liquids What Does It Mean To Lower The Melting Point Melting point, temperature at which the solid and liquid forms of a pure substance can exist in equilibrium. It says something about the identity of a compound, and something about the purity of a. At the melting point, the solid and liquid states both exist and are at equilibrium. Impure solids melt at lower temperatures and may also melt over. What Does It Mean To Lower The Melting Point.
From byjus.com
What is the difference between melting point and boiling point What Does It Mean To Lower The Melting Point The melting point is usually defined as the point at which materials changes from a solid to a liquid. The first is for qualitative identification of the sample, and the second is for quantitative purity. When an impure solid is warmed, microscopic melting first occurs in a pure region by the component with the lower melting point. At the melting. What Does It Mean To Lower The Melting Point.
From www.youtube.com
Melting and Boiling Points p98 (Foundation p97) YouTube What Does It Mean To Lower The Melting Point The first is for qualitative identification of the sample, and the second is for quantitative purity. The temperature at which solid changes its state to liquid at atmospheric pressure is called. At the melting point, the solid and liquid states both exist and are at equilibrium. At the melting point, the change in gibbs free energy of a substance is. What Does It Mean To Lower The Melting Point.
From www.slideserve.com
PPT Theory of Melting Point PowerPoint Presentation, free download What Does It Mean To Lower The Melting Point The melting point is usually defined as the point at which materials changes from a solid to a liquid. The temperature at which solid changes its state to liquid at atmospheric pressure is called. Impure solids melt at lower temperatures and may also melt over a wider temperature range, known as melting point depression. The first is for qualitative identification. What Does It Mean To Lower The Melting Point.
From www.chemistrysteps.com
Boiling Point and Melting Point in Organic Chemistry Chemistry Steps What Does It Mean To Lower The Melting Point At the melting point, the change in gibbs free energy of a substance is zero, but the enthalpy and the entropy of the material are. The first is for qualitative identification of the sample, and the second is for quantitative purity. The melting point is usually defined as the point at which materials changes from a solid to a liquid.. What Does It Mean To Lower The Melting Point.
From byjus.com
36.Explain with reason the effect of impurities on the melting point What Does It Mean To Lower The Melting Point It says something about the identity of a compound, and something about the purity of a. At the melting point, the change in gibbs free energy of a substance is zero, but the enthalpy and the entropy of the material are. Impure solids melt at lower temperatures and may also melt over a wider temperature range, known as melting point. What Does It Mean To Lower The Melting Point.
From exylluyva.blob.core.windows.net
Solid Liquid Gas Melting Point Boiling Point at Margaret Chaffins blog What Does It Mean To Lower The Melting Point The melting point is usually defined as the point at which materials changes from a solid to a liquid. The temperature at which solid changes its state to liquid at atmospheric pressure is called. At the melting point, the change in gibbs free energy of a substance is zero, but the enthalpy and the entropy of the material are. At. What Does It Mean To Lower The Melting Point.
From www.slideserve.com
PPT Melting Point and Boiling Point PowerPoint Presentation, free What Does It Mean To Lower The Melting Point At the melting point, the change in gibbs free energy of a substance is zero, but the enthalpy and the entropy of the material are. There are two primary uses of melting point analysis data. When an impure solid is warmed, microscopic melting first occurs in a pure region by the component with the lower melting point. The temperature at. What Does It Mean To Lower The Melting Point.
From www.slideserve.com
PPT Chapter 16 “ Solutions ” PowerPoint Presentation, free download What Does It Mean To Lower The Melting Point The melting point of a compound is useful in two ways: When an impure solid is warmed, microscopic melting first occurs in a pure region by the component with the lower melting point. It says something about the identity of a compound, and something about the purity of a. At the melting point, the change in gibbs free energy of. What Does It Mean To Lower The Melting Point.
From www.slideserve.com
PPT Polarity and Intermolecular Forces PowerPoint Presentation, free What Does It Mean To Lower The Melting Point The melting point is the temperature at which a substance changes from a solid to a liquid. Melting point, temperature at which the solid and liquid forms of a pure substance can exist in equilibrium. The melting point of a compound is useful in two ways: At the melting point, the change in gibbs free energy of a substance is. What Does It Mean To Lower The Melting Point.
From exyaeabov.blob.core.windows.net
What Is The Boiling Point Melting Point And Freezing Point Of Water at What Does It Mean To Lower The Melting Point When an impure solid is warmed, microscopic melting first occurs in a pure region by the component with the lower melting point. There are two primary uses of melting point analysis data. The melting point is usually defined as the point at which materials changes from a solid to a liquid. The melting point of a compound is useful in. What Does It Mean To Lower The Melting Point.
From ksa.mytutorsource.com
Water Freezing Point Definition, Factors Affecting It & Supercooled What Does It Mean To Lower The Melting Point The melting point of a compound is useful in two ways: When an impure solid is warmed, microscopic melting first occurs in a pure region by the component with the lower melting point. At the melting point, the solid and liquid states both exist and are at equilibrium. At the melting point, the change in gibbs free energy of a. What Does It Mean To Lower The Melting Point.
From www.youtube.com
Guide to Metal Melting Points (ºF) YouTube What Does It Mean To Lower The Melting Point At the melting point, the solid and liquid states both exist and are at equilibrium. There are two primary uses of melting point analysis data. The melting point is the temperature at which a substance changes from a solid to a liquid. It says something about the identity of a compound, and something about the purity of a. At the. What Does It Mean To Lower The Melting Point.
From www.youtube.com
COMPARISON OF MELTING POINT YouTube What Does It Mean To Lower The Melting Point There are two primary uses of melting point analysis data. It says something about the identity of a compound, and something about the purity of a. Impure solids melt at lower temperatures and may also melt over a wider temperature range, known as melting point depression. When an impure solid is warmed, microscopic melting first occurs in a pure region. What Does It Mean To Lower The Melting Point.
From loeaanczy.blob.core.windows.net
Chlorine Boiling Point And Melting Point at Jane Gibbs blog What Does It Mean To Lower The Melting Point The temperature at which solid changes its state to liquid at atmospheric pressure is called. The melting point is the temperature at which a substance changes from a solid to a liquid. There are two primary uses of melting point analysis data. Impure solids melt at lower temperatures and may also melt over a wider temperature range, known as melting. What Does It Mean To Lower The Melting Point.
From www.chemistrysteps.com
Boiling Point and Melting Point in Organic Chemistry Chemistry Steps What Does It Mean To Lower The Melting Point When an impure solid is warmed, microscopic melting first occurs in a pure region by the component with the lower melting point. At the melting point, the solid and liquid states both exist and are at equilibrium. It says something about the identity of a compound, and something about the purity of a. At the melting point, the change in. What Does It Mean To Lower The Melting Point.
From loeaanczy.blob.core.windows.net
Chlorine Boiling Point And Melting Point at Jane Gibbs blog What Does It Mean To Lower The Melting Point There are two primary uses of melting point analysis data. At the melting point, the solid and liquid states both exist and are at equilibrium. The melting point is usually defined as the point at which materials changes from a solid to a liquid. When an impure solid is warmed, microscopic melting first occurs in a pure region by the. What Does It Mean To Lower The Melting Point.
From www.slideserve.com
PPT MELTING AND BOILING PowerPoint Presentation, free download ID What Does It Mean To Lower The Melting Point At the melting point, the change in gibbs free energy of a substance is zero, but the enthalpy and the entropy of the material are. The melting point is usually defined as the point at which materials changes from a solid to a liquid. The temperature at which solid changes its state to liquid at atmospheric pressure is called. The. What Does It Mean To Lower The Melting Point.
From www.slideserve.com
PPT Theory of Melting Point PowerPoint Presentation, free download What Does It Mean To Lower The Melting Point The temperature at which solid changes its state to liquid at atmospheric pressure is called. There are two primary uses of melting point analysis data. At the melting point, the change in gibbs free energy of a substance is zero, but the enthalpy and the entropy of the material are. It says something about the identity of a compound, and. What Does It Mean To Lower The Melting Point.
From www.worksheetsplanet.com
What is Melting Point Definition of Melting Point What Does It Mean To Lower The Melting Point The first is for qualitative identification of the sample, and the second is for quantitative purity. At the melting point, the change in gibbs free energy of a substance is zero, but the enthalpy and the entropy of the material are. When an impure solid is warmed, microscopic melting first occurs in a pure region by the component with the. What Does It Mean To Lower The Melting Point.
From www.museoinclusivo.com
What is Aluminum’s Melting Point? A Comprehensive Guide Aluminum What Does It Mean To Lower The Melting Point Melting point, temperature at which the solid and liquid forms of a pure substance can exist in equilibrium. The temperature at which solid changes its state to liquid at atmospheric pressure is called. Impure solids melt at lower temperatures and may also melt over a wider temperature range, known as melting point depression. The melting point is usually defined as. What Does It Mean To Lower The Melting Point.
From circuitlibbottega.z21.web.core.windows.net
How To Determine Melting Point Chemistry What Does It Mean To Lower The Melting Point When an impure solid is warmed, microscopic melting first occurs in a pure region by the component with the lower melting point. The melting point of a compound is useful in two ways: The melting point is usually defined as the point at which materials changes from a solid to a liquid. The first is for qualitative identification of the. What Does It Mean To Lower The Melting Point.
From www.slideserve.com
PPT Theory of Melting Point PowerPoint Presentation ID310369 What Does It Mean To Lower The Melting Point It says something about the identity of a compound, and something about the purity of a. There are two primary uses of melting point analysis data. The melting point is the temperature at which a substance changes from a solid to a liquid. The melting point is usually defined as the point at which materials changes from a solid to. What Does It Mean To Lower The Melting Point.
From www.slideserve.com
PPT Water PowerPoint Presentation, free download ID6551434 What Does It Mean To Lower The Melting Point There are two primary uses of melting point analysis data. The melting point is usually defined as the point at which materials changes from a solid to a liquid. The first is for qualitative identification of the sample, and the second is for quantitative purity. Impure solids melt at lower temperatures and may also melt over a wider temperature range,. What Does It Mean To Lower The Melting Point.
From www.slideserve.com
PPT Theory of Melting Point PowerPoint Presentation, free download What Does It Mean To Lower The Melting Point At the melting point, the change in gibbs free energy of a substance is zero, but the enthalpy and the entropy of the material are. The temperature at which solid changes its state to liquid at atmospheric pressure is called. When an impure solid is warmed, microscopic melting first occurs in a pure region by the component with the lower. What Does It Mean To Lower The Melting Point.
From www.slideserve.com
PPT MELTING AND BOILING PowerPoint Presentation, free download ID What Does It Mean To Lower The Melting Point There are two primary uses of melting point analysis data. The first is for qualitative identification of the sample, and the second is for quantitative purity. The melting point of a compound is useful in two ways: Impure solids melt at lower temperatures and may also melt over a wider temperature range, known as melting point depression. The melting point. What Does It Mean To Lower The Melting Point.
From msestudent.com
What are Alloys? (Definition, Examples, and Metallurgy) Materials What Does It Mean To Lower The Melting Point The melting point is usually defined as the point at which materials changes from a solid to a liquid. There are two primary uses of melting point analysis data. It says something about the identity of a compound, and something about the purity of a. At the melting point, the solid and liquid states both exist and are at equilibrium.. What Does It Mean To Lower The Melting Point.
From exylluyva.blob.core.windows.net
Solid Liquid Gas Melting Point Boiling Point at Margaret Chaffins blog What Does It Mean To Lower The Melting Point The melting point of a compound is useful in two ways: There are two primary uses of melting point analysis data. Melting point, temperature at which the solid and liquid forms of a pure substance can exist in equilibrium. Impure solids melt at lower temperatures and may also melt over a wider temperature range, known as melting point depression. It. What Does It Mean To Lower The Melting Point.