Aluminum Hydroxide + Hcl . For each element, we check if the number of atoms is balanced on both sides of the. aluminum reacts with diluted hydrochloric acid at room temperature. The metal dissolves in hydrochloric acid, yielding aluminum chloride and colorless hydrogen gas. 1 al (oh) 3 + 1 hcl = 1 alcl 3 + 1 h 2 o. The aluminum hydroxide tends to cause. several antacids have aluminum hydroxide, al(oh) 3, as an active ingredient. Balance any equation or reaction using this chemical equation balancer! there are three main steps for writing the net ionic equation for al (oh)3 + hcl = alcl3 + h2o (aluminum hydroxide +. This reaction is irreversible, as the final products will not react with each other. Find out what type of. in this video we'll balance the equation al (oh)3 + hcl = alcl3 + h2o. the balanced equation shows the reaction between aluminium hydroxide and hydrochloric acid to form aluminium. how to balance equations.
from science-direct-topics.blogspot.com
several antacids have aluminum hydroxide, al(oh) 3, as an active ingredient. in this video we'll balance the equation al (oh)3 + hcl = alcl3 + h2o. there are three main steps for writing the net ionic equation for al (oh)3 + hcl = alcl3 + h2o (aluminum hydroxide +. Balance any equation or reaction using this chemical equation balancer! how to balance equations. The metal dissolves in hydrochloric acid, yielding aluminum chloride and colorless hydrogen gas. 1 al (oh) 3 + 1 hcl = 1 alcl 3 + 1 h 2 o. The aluminum hydroxide tends to cause. For each element, we check if the number of atoms is balanced on both sides of the. This reaction is irreversible, as the final products will not react with each other.
Science Direct Topics Aluminum Hydrochloric Acid
Aluminum Hydroxide + Hcl Find out what type of. 1 al (oh) 3 + 1 hcl = 1 alcl 3 + 1 h 2 o. in this video we'll balance the equation al (oh)3 + hcl = alcl3 + h2o. Balance any equation or reaction using this chemical equation balancer! there are three main steps for writing the net ionic equation for al (oh)3 + hcl = alcl3 + h2o (aluminum hydroxide +. the balanced equation shows the reaction between aluminium hydroxide and hydrochloric acid to form aluminium. Find out what type of. how to balance equations. The metal dissolves in hydrochloric acid, yielding aluminum chloride and colorless hydrogen gas. The aluminum hydroxide tends to cause. For each element, we check if the number of atoms is balanced on both sides of the. aluminum reacts with diluted hydrochloric acid at room temperature. several antacids have aluminum hydroxide, al(oh) 3, as an active ingredient. This reaction is irreversible, as the final products will not react with each other.
From www.numerade.com
SOLVED From the following balanced equation, the mass of aluminum Aluminum Hydroxide + Hcl The aluminum hydroxide tends to cause. This reaction is irreversible, as the final products will not react with each other. how to balance equations. aluminum reacts with diluted hydrochloric acid at room temperature. Find out what type of. the balanced equation shows the reaction between aluminium hydroxide and hydrochloric acid to form aluminium. 1 al (oh). Aluminum Hydroxide + Hcl.
From aluminumhydroxidesaidan.blogspot.com
Aluminum Hydroxide Balanced Equation For Aluminum Hydroxide And Aluminum Hydroxide + Hcl For each element, we check if the number of atoms is balanced on both sides of the. in this video we'll balance the equation al (oh)3 + hcl = alcl3 + h2o. This reaction is irreversible, as the final products will not react with each other. The aluminum hydroxide tends to cause. there are three main steps for. Aluminum Hydroxide + Hcl.
From www.sodium-cryolite.com
Industrial Grade Aluminium Hydroxide Powder Insoluble In Water Aluminum Hydroxide + Hcl Find out what type of. For each element, we check if the number of atoms is balanced on both sides of the. several antacids have aluminum hydroxide, al(oh) 3, as an active ingredient. This reaction is irreversible, as the final products will not react with each other. the balanced equation shows the reaction between aluminium hydroxide and hydrochloric. Aluminum Hydroxide + Hcl.
From www.indiamart.com
SRL Aluminium Hydroxide Dried Gel at best price in Ranchi by Lab Mate Aluminum Hydroxide + Hcl This reaction is irreversible, as the final products will not react with each other. aluminum reacts with diluted hydrochloric acid at room temperature. Find out what type of. how to balance equations. several antacids have aluminum hydroxide, al(oh) 3, as an active ingredient. in this video we'll balance the equation al (oh)3 + hcl = alcl3. Aluminum Hydroxide + Hcl.
From www.youtube.com
Hydrochloric Acid Reacting With Aluminum YouTube Aluminum Hydroxide + Hcl aluminum reacts with diluted hydrochloric acid at room temperature. the balanced equation shows the reaction between aluminium hydroxide and hydrochloric acid to form aluminium. The metal dissolves in hydrochloric acid, yielding aluminum chloride and colorless hydrogen gas. This reaction is irreversible, as the final products will not react with each other. The aluminum hydroxide tends to cause. For. Aluminum Hydroxide + Hcl.
From www.numerade.com
SOLVED(d) Aluminum hydroxide is an over the counterantacid that reacts Aluminum Hydroxide + Hcl For each element, we check if the number of atoms is balanced on both sides of the. The aluminum hydroxide tends to cause. This reaction is irreversible, as the final products will not react with each other. the balanced equation shows the reaction between aluminium hydroxide and hydrochloric acid to form aluminium. 1 al (oh) 3 + 1. Aluminum Hydroxide + Hcl.
From brainly.in
9.Write the chemical formula (i) Hydrochloric acid (ii) Magnesium Aluminum Hydroxide + Hcl in this video we'll balance the equation al (oh)3 + hcl = alcl3 + h2o. several antacids have aluminum hydroxide, al(oh) 3, as an active ingredient. The metal dissolves in hydrochloric acid, yielding aluminum chloride and colorless hydrogen gas. This reaction is irreversible, as the final products will not react with each other. The aluminum hydroxide tends to. Aluminum Hydroxide + Hcl.
From fphoto.photoshelter.com
science chemistry exothermic reaction hydrochloric acid aluminum Aluminum Hydroxide + Hcl The aluminum hydroxide tends to cause. several antacids have aluminum hydroxide, al(oh) 3, as an active ingredient. the balanced equation shows the reaction between aluminium hydroxide and hydrochloric acid to form aluminium. This reaction is irreversible, as the final products will not react with each other. The metal dissolves in hydrochloric acid, yielding aluminum chloride and colorless hydrogen. Aluminum Hydroxide + Hcl.
From science-direct-topics.blogspot.com
Science Direct Topics Aluminum Hydrochloric Acid Aluminum Hydroxide + Hcl Balance any equation or reaction using this chemical equation balancer! how to balance equations. the balanced equation shows the reaction between aluminium hydroxide and hydrochloric acid to form aluminium. in this video we'll balance the equation al (oh)3 + hcl = alcl3 + h2o. The metal dissolves in hydrochloric acid, yielding aluminum chloride and colorless hydrogen gas.. Aluminum Hydroxide + Hcl.
From www.numerade.com
SOLVEDThe chemistry of gallium (a) Gallium hydroxide, like aluminum Aluminum Hydroxide + Hcl Find out what type of. how to balance equations. there are three main steps for writing the net ionic equation for al (oh)3 + hcl = alcl3 + h2o (aluminum hydroxide +. aluminum reacts with diluted hydrochloric acid at room temperature. This reaction is irreversible, as the final products will not react with each other. For each. Aluminum Hydroxide + Hcl.
From www.imxhbio.com
Aluminum Hydroxide Gel Aluminum Hydroxide + Hcl For each element, we check if the number of atoms is balanced on both sides of the. several antacids have aluminum hydroxide, al(oh) 3, as an active ingredient. Find out what type of. Balance any equation or reaction using this chemical equation balancer! how to balance equations. the balanced equation shows the reaction between aluminium hydroxide and. Aluminum Hydroxide + Hcl.
From www.mcguffmedical.com
Aluminum Hydroxide Gel, Oral Suspension, 320mg/5mL, 16oz Bottle Aluminum Hydroxide + Hcl The aluminum hydroxide tends to cause. The metal dissolves in hydrochloric acid, yielding aluminum chloride and colorless hydrogen gas. aluminum reacts with diluted hydrochloric acid at room temperature. how to balance equations. This reaction is irreversible, as the final products will not react with each other. 1 al (oh) 3 + 1 hcl = 1 alcl 3. Aluminum Hydroxide + Hcl.
From aluminumhydroxidesaidan.blogspot.com
Aluminum Hydroxide Formation Of Aluminum Hydroxide Aluminum Hydroxide + Hcl Balance any equation or reaction using this chemical equation balancer! there are three main steps for writing the net ionic equation for al (oh)3 + hcl = alcl3 + h2o (aluminum hydroxide +. aluminum reacts with diluted hydrochloric acid at room temperature. in this video we'll balance the equation al (oh)3 + hcl = alcl3 + h2o.. Aluminum Hydroxide + Hcl.
From www.hmicronpowder.com
Aluminum Hydroxide Chemical Industries Hosokawa Micron Powder Systems Aluminum Hydroxide + Hcl there are three main steps for writing the net ionic equation for al (oh)3 + hcl = alcl3 + h2o (aluminum hydroxide +. This reaction is irreversible, as the final products will not react with each other. 1 al (oh) 3 + 1 hcl = 1 alcl 3 + 1 h 2 o. the balanced equation shows. Aluminum Hydroxide + Hcl.
From www.indiamart.com
Aluminium Hydroxide, Magnesium HCL and Simethicone Oral Suspension at Aluminum Hydroxide + Hcl aluminum reacts with diluted hydrochloric acid at room temperature. there are three main steps for writing the net ionic equation for al (oh)3 + hcl = alcl3 + h2o (aluminum hydroxide +. 1 al (oh) 3 + 1 hcl = 1 alcl 3 + 1 h 2 o. several antacids have aluminum hydroxide, al(oh) 3, as. Aluminum Hydroxide + Hcl.
From www.sodium-cryolite.com
95 Odorless Aluminium Hydroxide Powder Hydrochloric Acid Soluble Aluminum Hydroxide + Hcl Find out what type of. The aluminum hydroxide tends to cause. there are three main steps for writing the net ionic equation for al (oh)3 + hcl = alcl3 + h2o (aluminum hydroxide +. The metal dissolves in hydrochloric acid, yielding aluminum chloride and colorless hydrogen gas. 1 al (oh) 3 + 1 hcl = 1 alcl 3. Aluminum Hydroxide + Hcl.
From www.numerade.com
SOLVED Aluminum hydroxide can be produced by the following reaction in Aluminum Hydroxide + Hcl 1 al (oh) 3 + 1 hcl = 1 alcl 3 + 1 h 2 o. For each element, we check if the number of atoms is balanced on both sides of the. This reaction is irreversible, as the final products will not react with each other. how to balance equations. in this video we'll balance the. Aluminum Hydroxide + Hcl.
From www.numerade.com
SOLVED Be sure to answer all parts. Some liquid antacids contain Aluminum Hydroxide + Hcl the balanced equation shows the reaction between aluminium hydroxide and hydrochloric acid to form aluminium. The metal dissolves in hydrochloric acid, yielding aluminum chloride and colorless hydrogen gas. in this video we'll balance the equation al (oh)3 + hcl = alcl3 + h2o. there are three main steps for writing the net ionic equation for al (oh)3. Aluminum Hydroxide + Hcl.
From www.kroger.com
VARIES 001PD320480 Aluminum Hydroxide Gel 320 mg x 473 ml, 1 Kroger Aluminum Hydroxide + Hcl in this video we'll balance the equation al (oh)3 + hcl = alcl3 + h2o. there are three main steps for writing the net ionic equation for al (oh)3 + hcl = alcl3 + h2o (aluminum hydroxide +. For each element, we check if the number of atoms is balanced on both sides of the. several antacids. Aluminum Hydroxide + Hcl.
From w20.b2m.cz
Al Hcl Alcl3 H2 EDUCA Aluminum Hydroxide + Hcl For each element, we check if the number of atoms is balanced on both sides of the. there are three main steps for writing the net ionic equation for al (oh)3 + hcl = alcl3 + h2o (aluminum hydroxide +. Find out what type of. several antacids have aluminum hydroxide, al(oh) 3, as an active ingredient. This reaction. Aluminum Hydroxide + Hcl.
From hydrogenchloridemekaiga.blogspot.com
Hydrogen Chloride Hydrogen Chloride Reaction With Aluminum Aluminum Hydroxide + Hcl how to balance equations. For each element, we check if the number of atoms is balanced on both sides of the. The metal dissolves in hydrochloric acid, yielding aluminum chloride and colorless hydrogen gas. several antacids have aluminum hydroxide, al(oh) 3, as an active ingredient. the balanced equation shows the reaction between aluminium hydroxide and hydrochloric acid. Aluminum Hydroxide + Hcl.
From science-direct-topics.blogspot.com
Science Direct Topics Aluminum Hydrochloric Acid Aluminum Hydroxide + Hcl The aluminum hydroxide tends to cause. Balance any equation or reaction using this chemical equation balancer! how to balance equations. For each element, we check if the number of atoms is balanced on both sides of the. aluminum reacts with diluted hydrochloric acid at room temperature. The metal dissolves in hydrochloric acid, yielding aluminum chloride and colorless hydrogen. Aluminum Hydroxide + Hcl.
From www.indiamart.com
Aluminium Hydroxide, Purity 90 , 25 Kg at Rs 55/kg in Vapi ID Aluminum Hydroxide + Hcl Balance any equation or reaction using this chemical equation balancer! 1 al (oh) 3 + 1 hcl = 1 alcl 3 + 1 h 2 o. several antacids have aluminum hydroxide, al(oh) 3, as an active ingredient. aluminum reacts with diluted hydrochloric acid at room temperature. how to balance equations. the balanced equation shows the. Aluminum Hydroxide + Hcl.
From spanish.aluminumhydroxidechemical.com
Sustancia química sólida blanca 21645512 del hidróxido de aluminio Aluminum Hydroxide + Hcl 1 al (oh) 3 + 1 hcl = 1 alcl 3 + 1 h 2 o. The metal dissolves in hydrochloric acid, yielding aluminum chloride and colorless hydrogen gas. several antacids have aluminum hydroxide, al(oh) 3, as an active ingredient. Find out what type of. aluminum reacts with diluted hydrochloric acid at room temperature. the balanced. Aluminum Hydroxide + Hcl.
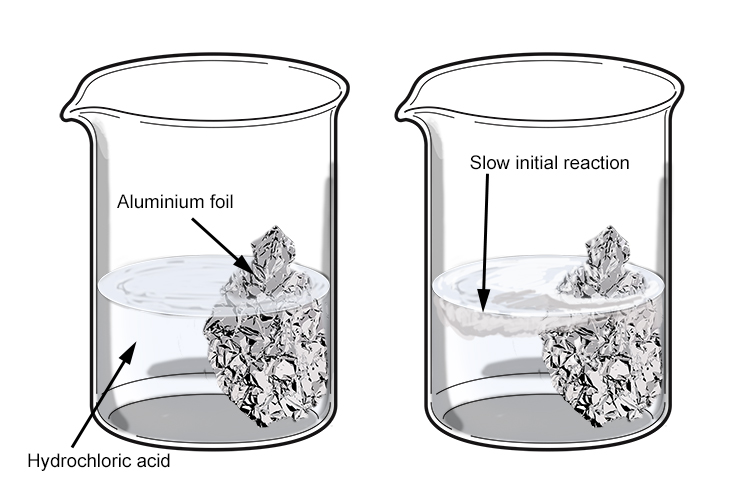
From mammothmemory.net
Aluminium reaction to hydrochloric acid is slow to start Aluminum Hydroxide + Hcl 1 al (oh) 3 + 1 hcl = 1 alcl 3 + 1 h 2 o. in this video we'll balance the equation al (oh)3 + hcl = alcl3 + h2o. there are three main steps for writing the net ionic equation for al (oh)3 + hcl = alcl3 + h2o (aluminum hydroxide +. Balance any equation. Aluminum Hydroxide + Hcl.
From hydrogenchloridemekaiga.blogspot.com
Hydrogen Chloride Hydrogen Chloride Reaction With Aluminum Aluminum Hydroxide + Hcl Balance any equation or reaction using this chemical equation balancer! For each element, we check if the number of atoms is balanced on both sides of the. in this video we'll balance the equation al (oh)3 + hcl = alcl3 + h2o. The metal dissolves in hydrochloric acid, yielding aluminum chloride and colorless hydrogen gas. aluminum reacts with. Aluminum Hydroxide + Hcl.
From fphoto.photoshelter.com
science chemistry exothermic reaction hydrochloric acid aluminum Aluminum Hydroxide + Hcl 1 al (oh) 3 + 1 hcl = 1 alcl 3 + 1 h 2 o. in this video we'll balance the equation al (oh)3 + hcl = alcl3 + h2o. The metal dissolves in hydrochloric acid, yielding aluminum chloride and colorless hydrogen gas. For each element, we check if the number of atoms is balanced on both. Aluminum Hydroxide + Hcl.
From www.aluminumhydroxidechemical.com
Hydrochloric Acid Soluble 95 Aluminum Hydroxide Chemical Aluminum Hydroxide + Hcl there are three main steps for writing the net ionic equation for al (oh)3 + hcl = alcl3 + h2o (aluminum hydroxide +. in this video we'll balance the equation al (oh)3 + hcl = alcl3 + h2o. Balance any equation or reaction using this chemical equation balancer! This reaction is irreversible, as the final products will not. Aluminum Hydroxide + Hcl.
From www.carolina.com
Aluminum Hydroxide, Anhydrous, Laboratory Grade, 500 g Aluminum Hydroxide + Hcl several antacids have aluminum hydroxide, al(oh) 3, as an active ingredient. 1 al (oh) 3 + 1 hcl = 1 alcl 3 + 1 h 2 o. in this video we'll balance the equation al (oh)3 + hcl = alcl3 + h2o. aluminum reacts with diluted hydrochloric acid at room temperature. For each element, we check. Aluminum Hydroxide + Hcl.
From www.youtube.com
How to Balance Al(OH)3 + HCl = AlCl3 + H2O Aluminum hydroxide Aluminum Hydroxide + Hcl in this video we'll balance the equation al (oh)3 + hcl = alcl3 + h2o. This reaction is irreversible, as the final products will not react with each other. For each element, we check if the number of atoms is balanced on both sides of the. there are three main steps for writing the net ionic equation for. Aluminum Hydroxide + Hcl.
From www.youtube.com
Balancing Chemical Equations. Part 1 Aluminium and hydrochloric acid Aluminum Hydroxide + Hcl For each element, we check if the number of atoms is balanced on both sides of the. This reaction is irreversible, as the final products will not react with each other. there are three main steps for writing the net ionic equation for al (oh)3 + hcl = alcl3 + h2o (aluminum hydroxide +. The metal dissolves in hydrochloric. Aluminum Hydroxide + Hcl.
From www.youtube.com
Hydrogen Production using hydrochloric acid and aluminum YouTube Aluminum Hydroxide + Hcl 1 al (oh) 3 + 1 hcl = 1 alcl 3 + 1 h 2 o. there are three main steps for writing the net ionic equation for al (oh)3 + hcl = alcl3 + h2o (aluminum hydroxide +. Find out what type of. how to balance equations. the balanced equation shows the reaction between aluminium. Aluminum Hydroxide + Hcl.
From www.numerade.com
SOLVEDaluminium metal strip is added in hydrochloric acid to produce Aluminum Hydroxide + Hcl in this video we'll balance the equation al (oh)3 + hcl = alcl3 + h2o. For each element, we check if the number of atoms is balanced on both sides of the. This reaction is irreversible, as the final products will not react with each other. several antacids have aluminum hydroxide, al(oh) 3, as an active ingredient. . Aluminum Hydroxide + Hcl.
From hargravesotc.com
Aluminum Hydroxide Gel For Heartburn Symptoms Hargraves Online Aluminum Hydroxide + Hcl Balance any equation or reaction using this chemical equation balancer! Find out what type of. how to balance equations. there are three main steps for writing the net ionic equation for al (oh)3 + hcl = alcl3 + h2o (aluminum hydroxide +. the balanced equation shows the reaction between aluminium hydroxide and hydrochloric acid to form aluminium.. Aluminum Hydroxide + Hcl.
From slideplayer.com
DRUGS USED IN GIT. ppt download Aluminum Hydroxide + Hcl The metal dissolves in hydrochloric acid, yielding aluminum chloride and colorless hydrogen gas. several antacids have aluminum hydroxide, al(oh) 3, as an active ingredient. the balanced equation shows the reaction between aluminium hydroxide and hydrochloric acid to form aluminium. 1 al (oh) 3 + 1 hcl = 1 alcl 3 + 1 h 2 o. aluminum. Aluminum Hydroxide + Hcl.