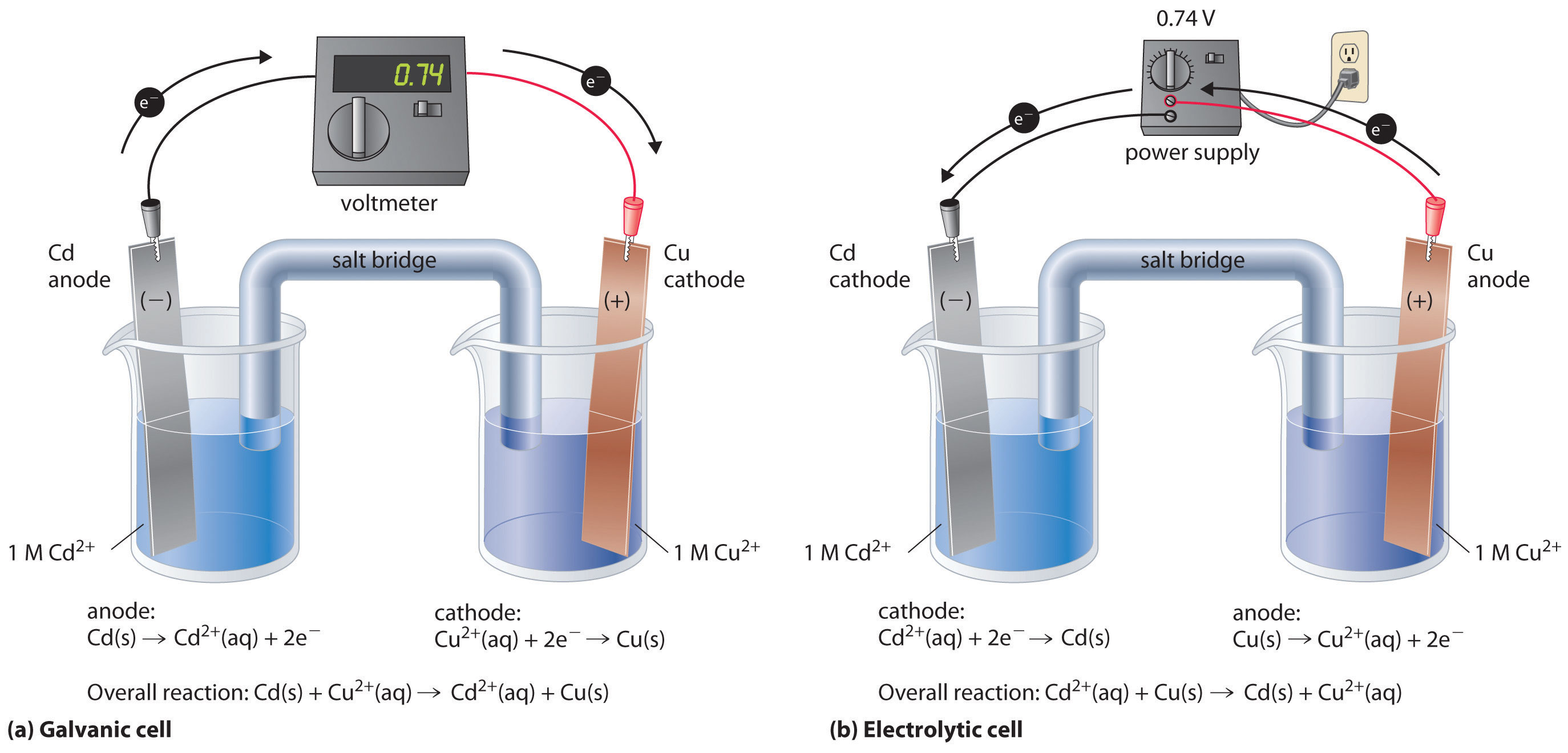
Electrolysis Is Quizlet . The ions are forced to. study with quizlet and memorize flashcards containing terms like what is electrolysis, what is an electrode,. study with quizlet and memorize flashcards containing terms like what is the definition of electrolysis?, what must happen for. electrolysis is the process of using electricity to break down or decompose a compound (usually an ionic compound in. in electrolytic cells, electrical energy causes nonspontaneous reactions to occur in a process known as electrolysis. Electrolysis can also be used to. study with quizlet and memorize flashcards containing terms like what are electrolytes?, what is electrolysis?, what is a cathode?. electrolysis involves passing an electric current through either a molten salt or an ionic solution. in electrolysis, an external voltage is applied to drive a nonspontaneous reaction. during electrolysis \(\ce{h2(g)}\) and \(\ce{cl2(g)}\) bubble from the cathode and anode, respectively.
from webmis.highland.cc.il.us
in electrolysis, an external voltage is applied to drive a nonspontaneous reaction. Electrolysis can also be used to. The ions are forced to. electrolysis involves passing an electric current through either a molten salt or an ionic solution. during electrolysis \(\ce{h2(g)}\) and \(\ce{cl2(g)}\) bubble from the cathode and anode, respectively. study with quizlet and memorize flashcards containing terms like what is the definition of electrolysis?, what must happen for. study with quizlet and memorize flashcards containing terms like what are electrolytes?, what is electrolysis?, what is a cathode?. in electrolytic cells, electrical energy causes nonspontaneous reactions to occur in a process known as electrolysis. electrolysis is the process of using electricity to break down or decompose a compound (usually an ionic compound in. study with quizlet and memorize flashcards containing terms like what is electrolysis, what is an electrode,.
Electrolysis
Electrolysis Is Quizlet Electrolysis can also be used to. in electrolytic cells, electrical energy causes nonspontaneous reactions to occur in a process known as electrolysis. in electrolysis, an external voltage is applied to drive a nonspontaneous reaction. electrolysis is the process of using electricity to break down or decompose a compound (usually an ionic compound in. electrolysis involves passing an electric current through either a molten salt or an ionic solution. Electrolysis can also be used to. during electrolysis \(\ce{h2(g)}\) and \(\ce{cl2(g)}\) bubble from the cathode and anode, respectively. The ions are forced to. study with quizlet and memorize flashcards containing terms like what is electrolysis, what is an electrode,. study with quizlet and memorize flashcards containing terms like what are electrolytes?, what is electrolysis?, what is a cathode?. study with quizlet and memorize flashcards containing terms like what is the definition of electrolysis?, what must happen for.
From greenenergymaterial.com
What Is Electrolysis » Green Energy Material Electrolysis Is Quizlet in electrolytic cells, electrical energy causes nonspontaneous reactions to occur in a process known as electrolysis. The ions are forced to. study with quizlet and memorize flashcards containing terms like what is electrolysis, what is an electrode,. electrolysis is the process of using electricity to break down or decompose a compound (usually an ionic compound in. . Electrolysis Is Quizlet.
From www.professionalimageenhancement.com
Professional Image Enhancement Skin Care & Electrolysis— Is Electrolysis Is Quizlet during electrolysis \(\ce{h2(g)}\) and \(\ce{cl2(g)}\) bubble from the cathode and anode, respectively. The ions are forced to. electrolysis is the process of using electricity to break down or decompose a compound (usually an ionic compound in. electrolysis involves passing an electric current through either a molten salt or an ionic solution. in electrolytic cells, electrical energy. Electrolysis Is Quizlet.
From quizizz.com
50+ electrolysis and faradays law worksheets on Quizizz Free & Printable Electrolysis Is Quizlet in electrolytic cells, electrical energy causes nonspontaneous reactions to occur in a process known as electrolysis. study with quizlet and memorize flashcards containing terms like what is electrolysis, what is an electrode,. Electrolysis can also be used to. The ions are forced to. electrolysis is the process of using electricity to break down or decompose a compound. Electrolysis Is Quizlet.
From www.electricalvolt.com
Faraday's Law of electrolysis Electrolysis Is Quizlet in electrolysis, an external voltage is applied to drive a nonspontaneous reaction. study with quizlet and memorize flashcards containing terms like what is the definition of electrolysis?, what must happen for. electrolysis is the process of using electricity to break down or decompose a compound (usually an ionic compound in. study with quizlet and memorize flashcards. Electrolysis Is Quizlet.
From quizlet.com
Unit 10 Electrolysis Diagram Quizlet Electrolysis Is Quizlet Electrolysis can also be used to. study with quizlet and memorize flashcards containing terms like what is the definition of electrolysis?, what must happen for. during electrolysis \(\ce{h2(g)}\) and \(\ce{cl2(g)}\) bubble from the cathode and anode, respectively. electrolysis involves passing an electric current through either a molten salt or an ionic solution. electrolysis is the process. Electrolysis Is Quizlet.
From www.quanswer.com
What is electrolysis? Quanswer Electrolysis Is Quizlet study with quizlet and memorize flashcards containing terms like what is the definition of electrolysis?, what must happen for. study with quizlet and memorize flashcards containing terms like what are electrolytes?, what is electrolysis?, what is a cathode?. in electrolysis, an external voltage is applied to drive a nonspontaneous reaction. Electrolysis can also be used to. . Electrolysis Is Quizlet.
From elispot.biz
Electrolysis side effects 5 Myths About Electrolysis Revealed Electrolysis Is Quizlet study with quizlet and memorize flashcards containing terms like what is the definition of electrolysis?, what must happen for. during electrolysis \(\ce{h2(g)}\) and \(\ce{cl2(g)}\) bubble from the cathode and anode, respectively. Electrolysis can also be used to. in electrolytic cells, electrical energy causes nonspontaneous reactions to occur in a process known as electrolysis. in electrolysis, an. Electrolysis Is Quizlet.
From quizlet.com
Chemistry Required Practical 3 Chemical Changes 'Investigate the Electrolysis Is Quizlet electrolysis is the process of using electricity to break down or decompose a compound (usually an ionic compound in. study with quizlet and memorize flashcards containing terms like what is electrolysis, what is an electrode,. The ions are forced to. study with quizlet and memorize flashcards containing terms like what are electrolytes?, what is electrolysis?, what is. Electrolysis Is Quizlet.
From howelectrical.com
What is Electrolysis? Definition, Process, Diagram & Law Electrical Electrolysis Is Quizlet in electrolytic cells, electrical energy causes nonspontaneous reactions to occur in a process known as electrolysis. study with quizlet and memorize flashcards containing terms like what are electrolytes?, what is electrolysis?, what is a cathode?. in electrolysis, an external voltage is applied to drive a nonspontaneous reaction. Electrolysis can also be used to. during electrolysis \(\ce{h2(g)}\). Electrolysis Is Quizlet.
From pricklynomore.com
The science of electrolysis — Prickly Pear Electrolysis & Skincare Electrolysis Is Quizlet study with quizlet and memorize flashcards containing terms like what is the definition of electrolysis?, what must happen for. Electrolysis can also be used to. electrolysis involves passing an electric current through either a molten salt or an ionic solution. The ions are forced to. study with quizlet and memorize flashcards containing terms like what is electrolysis,. Electrolysis Is Quizlet.
From quizlet.com
Chemistry Electrolysis Electrolysis of Solutions Diagram Quizlet Electrolysis Is Quizlet during electrolysis \(\ce{h2(g)}\) and \(\ce{cl2(g)}\) bubble from the cathode and anode, respectively. study with quizlet and memorize flashcards containing terms like what is the definition of electrolysis?, what must happen for. in electrolysis, an external voltage is applied to drive a nonspontaneous reaction. study with quizlet and memorize flashcards containing terms like what is electrolysis, what. Electrolysis Is Quizlet.
From www.toppr.com
Electrolysis of concentrated aqueous solution of a compound gave C2H6 Electrolysis Is Quizlet electrolysis is the process of using electricity to break down or decompose a compound (usually an ionic compound in. study with quizlet and memorize flashcards containing terms like what are electrolytes?, what is electrolysis?, what is a cathode?. electrolysis involves passing an electric current through either a molten salt or an ionic solution. study with quizlet. Electrolysis Is Quizlet.
From www.freepik.com
Premium Vector Electrolysis process vector illustration diagram Electrolysis Is Quizlet electrolysis is the process of using electricity to break down or decompose a compound (usually an ionic compound in. in electrolytic cells, electrical energy causes nonspontaneous reactions to occur in a process known as electrolysis. The ions are forced to. Electrolysis can also be used to. electrolysis involves passing an electric current through either a molten salt. Electrolysis Is Quizlet.
From quizlet.com
CC10 Electrolysis diagram Diagram Quizlet Electrolysis Is Quizlet in electrolytic cells, electrical energy causes nonspontaneous reactions to occur in a process known as electrolysis. study with quizlet and memorize flashcards containing terms like what are electrolytes?, what is electrolysis?, what is a cathode?. electrolysis involves passing an electric current through either a molten salt or an ionic solution. The ions are forced to. electrolysis. Electrolysis Is Quizlet.
From www.youtube.com
UNDERSTAND ELECTROLYSIS by looking at a few more examples. YouTube Electrolysis Is Quizlet electrolysis involves passing an electric current through either a molten salt or an ionic solution. The ions are forced to. study with quizlet and memorize flashcards containing terms like what is the definition of electrolysis?, what must happen for. electrolysis is the process of using electricity to break down or decompose a compound (usually an ionic compound. Electrolysis Is Quizlet.
From www.waivio.com
Application of electrolysis Electrolysis Is Quizlet in electrolysis, an external voltage is applied to drive a nonspontaneous reaction. study with quizlet and memorize flashcards containing terms like what are electrolytes?, what is electrolysis?, what is a cathode?. study with quizlet and memorize flashcards containing terms like what is the definition of electrolysis?, what must happen for. study with quizlet and memorize flashcards. Electrolysis Is Quizlet.
From quizlet.com
U5 Electrolysis golden. Diagram Quizlet Electrolysis Is Quizlet The ions are forced to. Electrolysis can also be used to. electrolysis involves passing an electric current through either a molten salt or an ionic solution. in electrolysis, an external voltage is applied to drive a nonspontaneous reaction. study with quizlet and memorize flashcards containing terms like what are electrolytes?, what is electrolysis?, what is a cathode?.. Electrolysis Is Quizlet.
From quizlet.com
Chemistry C4 Chemical changes and Electrolysis Diagram Quizlet Electrolysis Is Quizlet electrolysis involves passing an electric current through either a molten salt or an ionic solution. Electrolysis can also be used to. study with quizlet and memorize flashcards containing terms like what are electrolytes?, what is electrolysis?, what is a cathode?. electrolysis is the process of using electricity to break down or decompose a compound (usually an ionic. Electrolysis Is Quizlet.
From webmis.highland.cc.il.us
Electrolysis Electrolysis Is Quizlet study with quizlet and memorize flashcards containing terms like what are electrolytes?, what is electrolysis?, what is a cathode?. in electrolytic cells, electrical energy causes nonspontaneous reactions to occur in a process known as electrolysis. study with quizlet and memorize flashcards containing terms like what is the definition of electrolysis?, what must happen for. during electrolysis. Electrolysis Is Quizlet.
From chemistryskills.com
Electrolysis Chemistry Chemistry Skills Electrolysis Is Quizlet during electrolysis \(\ce{h2(g)}\) and \(\ce{cl2(g)}\) bubble from the cathode and anode, respectively. The ions are forced to. in electrolysis, an external voltage is applied to drive a nonspontaneous reaction. in electrolytic cells, electrical energy causes nonspontaneous reactions to occur in a process known as electrolysis. study with quizlet and memorize flashcards containing terms like what is. Electrolysis Is Quizlet.
From general.chemistrysteps.com
Electrolysis of Water Chemistry Steps Electrolysis Is Quizlet in electrolysis, an external voltage is applied to drive a nonspontaneous reaction. electrolysis is the process of using electricity to break down or decompose a compound (usually an ionic compound in. in electrolytic cells, electrical energy causes nonspontaneous reactions to occur in a process known as electrolysis. study with quizlet and memorize flashcards containing terms like. Electrolysis Is Quizlet.
From quizlet.com
Диаграмма Electrolysis Quizlet Electrolysis Is Quizlet study with quizlet and memorize flashcards containing terms like what is the definition of electrolysis?, what must happen for. in electrolysis, an external voltage is applied to drive a nonspontaneous reaction. The ions are forced to. during electrolysis \(\ce{h2(g)}\) and \(\ce{cl2(g)}\) bubble from the cathode and anode, respectively. electrolysis is the process of using electricity to. Electrolysis Is Quizlet.
From studylib.net
electrolysis worksheet Electrolysis Is Quizlet study with quizlet and memorize flashcards containing terms like what is electrolysis, what is an electrode,. during electrolysis \(\ce{h2(g)}\) and \(\ce{cl2(g)}\) bubble from the cathode and anode, respectively. electrolysis involves passing an electric current through either a molten salt or an ionic solution. study with quizlet and memorize flashcards containing terms like what are electrolytes?, what. Electrolysis Is Quizlet.
From www.savemyexams.co.uk
Electrolysis (5.3.1) CIE A Level Chemistry Revision Notes 2022 Save Electrolysis Is Quizlet in electrolytic cells, electrical energy causes nonspontaneous reactions to occur in a process known as electrolysis. Electrolysis can also be used to. The ions are forced to. electrolysis involves passing an electric current through either a molten salt or an ionic solution. during electrolysis \(\ce{h2(g)}\) and \(\ce{cl2(g)}\) bubble from the cathode and anode, respectively. study with. Electrolysis Is Quizlet.
From chemistrypage.in
Electrolysis Definition, Principle and Electrolytic Cell Electrolysis Is Quizlet in electrolysis, an external voltage is applied to drive a nonspontaneous reaction. study with quizlet and memorize flashcards containing terms like what is the definition of electrolysis?, what must happen for. study with quizlet and memorize flashcards containing terms like what are electrolytes?, what is electrolysis?, what is a cathode?. in electrolytic cells, electrical energy causes. Electrolysis Is Quizlet.
From quizlet.com
Electrolysis Chemistry Diagram Quizlet Electrolysis Is Quizlet study with quizlet and memorize flashcards containing terms like what is the definition of electrolysis?, what must happen for. study with quizlet and memorize flashcards containing terms like what is electrolysis, what is an electrode,. electrolysis is the process of using electricity to break down or decompose a compound (usually an ionic compound in. in electrolytic. Electrolysis Is Quizlet.
From byjus.com
Which is false about electrolysis? Electrolysis Is Quizlet during electrolysis \(\ce{h2(g)}\) and \(\ce{cl2(g)}\) bubble from the cathode and anode, respectively. study with quizlet and memorize flashcards containing terms like what is electrolysis, what is an electrode,. study with quizlet and memorize flashcards containing terms like what is the definition of electrolysis?, what must happen for. in electrolysis, an external voltage is applied to drive. Electrolysis Is Quizlet.
From quizlet.com
Chemistry Electrolysis Diagram Quizlet Electrolysis Is Quizlet electrolysis is the process of using electricity to break down or decompose a compound (usually an ionic compound in. study with quizlet and memorize flashcards containing terms like what are electrolytes?, what is electrolysis?, what is a cathode?. electrolysis involves passing an electric current through either a molten salt or an ionic solution. Electrolysis can also be. Electrolysis Is Quizlet.
From www.vecteezy.com
Electrolysis diagram experiment for education 1610466 Vector Art at Electrolysis Is Quizlet The ions are forced to. in electrolytic cells, electrical energy causes nonspontaneous reactions to occur in a process known as electrolysis. electrolysis is the process of using electricity to break down or decompose a compound (usually an ionic compound in. during electrolysis \(\ce{h2(g)}\) and \(\ce{cl2(g)}\) bubble from the cathode and anode, respectively. study with quizlet and. Electrolysis Is Quizlet.
From quizlet.com
Section 3 Equations, Calculations and Electrolysis Flashcards Quizlet Electrolysis Is Quizlet Electrolysis can also be used to. in electrolysis, an external voltage is applied to drive a nonspontaneous reaction. study with quizlet and memorize flashcards containing terms like what is the definition of electrolysis?, what must happen for. study with quizlet and memorize flashcards containing terms like what are electrolytes?, what is electrolysis?, what is a cathode?. . Electrolysis Is Quizlet.
From quizlet.com
GCSE Chemistry required practical activity Electrolysis Diagram Quizlet Electrolysis Is Quizlet in electrolysis, an external voltage is applied to drive a nonspontaneous reaction. during electrolysis \(\ce{h2(g)}\) and \(\ce{cl2(g)}\) bubble from the cathode and anode, respectively. study with quizlet and memorize flashcards containing terms like what is electrolysis, what is an electrode,. The ions are forced to. electrolysis involves passing an electric current through either a molten salt. Electrolysis Is Quizlet.
From quizlet.com
Electrolysis of aqueous solution Diagram Quizlet Electrolysis Is Quizlet The ions are forced to. electrolysis involves passing an electric current through either a molten salt or an ionic solution. electrolysis is the process of using electricity to break down or decompose a compound (usually an ionic compound in. during electrolysis \(\ce{h2(g)}\) and \(\ce{cl2(g)}\) bubble from the cathode and anode, respectively. study with quizlet and memorize. Electrolysis Is Quizlet.
From exyqzcdkp.blob.core.windows.net
Electrolysis In La at Katherine Winger blog Electrolysis Is Quizlet electrolysis involves passing an electric current through either a molten salt or an ionic solution. electrolysis is the process of using electricity to break down or decompose a compound (usually an ionic compound in. in electrolytic cells, electrical energy causes nonspontaneous reactions to occur in a process known as electrolysis. Electrolysis can also be used to. The. Electrolysis Is Quizlet.
From quizlet.com
Electrolysis Diagram Quizlet Electrolysis Is Quizlet The ions are forced to. Electrolysis can also be used to. during electrolysis \(\ce{h2(g)}\) and \(\ce{cl2(g)}\) bubble from the cathode and anode, respectively. study with quizlet and memorize flashcards containing terms like what is the definition of electrolysis?, what must happen for. in electrolytic cells, electrical energy causes nonspontaneous reactions to occur in a process known as. Electrolysis Is Quizlet.
From www.shutterstock.com
Electrolysis Technique That Uses Direct Electric Stock Vector (Royalty Electrolysis Is Quizlet The ions are forced to. during electrolysis \(\ce{h2(g)}\) and \(\ce{cl2(g)}\) bubble from the cathode and anode, respectively. in electrolytic cells, electrical energy causes nonspontaneous reactions to occur in a process known as electrolysis. in electrolysis, an external voltage is applied to drive a nonspontaneous reaction. study with quizlet and memorize flashcards containing terms like what is. Electrolysis Is Quizlet.