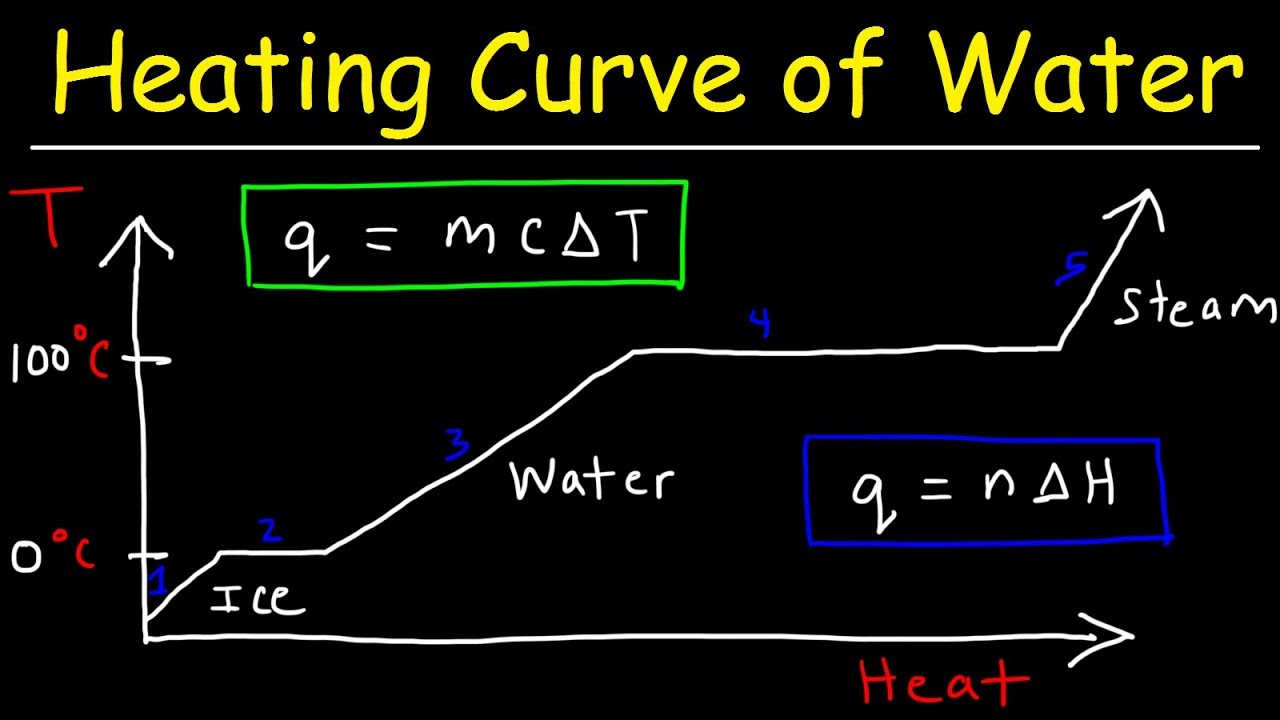
Cooling Curve Of Water Problem . 3 shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. For cooling curves, we start with the gas phase and remove heat energy. the energy changes that occur during phase changes can be quantified by using a heating or cooling curve. From the phase diagram for water, determine the state of water at:. a cooling curve is not exactly the reverse of the heating curve because many liquids do not freeze at the expected. The sample is initially ice at 1 atm and −23°c; As heat is added, the temperature of the ice increases linearly with time. Heating curves and phase changes (problems) page id. The water could then be. a cooling curve of a substance is a graph of the variation of the temperature with time as it is allowed to cool. for heating curves, we start with a solid and add heat energy. steam above 100°c could be steadily cooled down to 100°c, at which point it would condense to liquid water. The gradient of the cooling curve is related to.
from www.youtube.com
The gradient of the cooling curve is related to. the energy changes that occur during phase changes can be quantified by using a heating or cooling curve. Heating curves and phase changes (problems) page id. 3 shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. for heating curves, we start with a solid and add heat energy. For cooling curves, we start with the gas phase and remove heat energy. steam above 100°c could be steadily cooled down to 100°c, at which point it would condense to liquid water. a cooling curve of a substance is a graph of the variation of the temperature with time as it is allowed to cool. As heat is added, the temperature of the ice increases linearly with time. From the phase diagram for water, determine the state of water at:.
Heating Curve and Cooling Curve of Water Enthalpy of Fusion & Vaporization YouTube
Cooling Curve Of Water Problem a cooling curve of a substance is a graph of the variation of the temperature with time as it is allowed to cool. the energy changes that occur during phase changes can be quantified by using a heating or cooling curve. 3 shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. Heating curves and phase changes (problems) page id. The water could then be. As heat is added, the temperature of the ice increases linearly with time. For cooling curves, we start with the gas phase and remove heat energy. The sample is initially ice at 1 atm and −23°c; steam above 100°c could be steadily cooled down to 100°c, at which point it would condense to liquid water. From the phase diagram for water, determine the state of water at:. a cooling curve of a substance is a graph of the variation of the temperature with time as it is allowed to cool. The gradient of the cooling curve is related to. a cooling curve is not exactly the reverse of the heating curve because many liquids do not freeze at the expected. for heating curves, we start with a solid and add heat energy.
From brainly.com
Draw a cooling graph of water as it passes from 20°C to 20°C. The yaxis should be temperature Cooling Curve Of Water Problem steam above 100°c could be steadily cooled down to 100°c, at which point it would condense to liquid water. for heating curves, we start with a solid and add heat energy. Heating curves and phase changes (problems) page id. As heat is added, the temperature of the ice increases linearly with time. The gradient of the cooling curve. Cooling Curve Of Water Problem.
From learningzonegregorin2m.z4.web.core.windows.net
Heating Curve Of Water Explained Cooling Curve Of Water Problem a cooling curve of a substance is a graph of the variation of the temperature with time as it is allowed to cool. Heating curves and phase changes (problems) page id. the energy changes that occur during phase changes can be quantified by using a heating or cooling curve. for heating curves, we start with a solid. Cooling Curve Of Water Problem.
From learningschoolgraciauwb.z4.web.core.windows.net
Heating Curve Of Water Pdf Cooling Curve Of Water Problem 3 shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. steam above 100°c could be steadily cooled down to 100°c, at which point it would condense to liquid water. the energy changes that occur during phase changes can be quantified by using a heating or cooling curve. From the. Cooling Curve Of Water Problem.
From www.chegg.com
Solved The drawing below represents the cooling curve for Cooling Curve Of Water Problem a cooling curve is not exactly the reverse of the heating curve because many liquids do not freeze at the expected. a cooling curve of a substance is a graph of the variation of the temperature with time as it is allowed to cool. The sample is initially ice at 1 atm and −23°c; 3 shows a heating. Cooling Curve Of Water Problem.
From www.thinkswap.com
Cooling Curve of Water Physics Year 11 SACE Thinkswap Cooling Curve Of Water Problem For cooling curves, we start with the gas phase and remove heat energy. The water could then be. a cooling curve is not exactly the reverse of the heating curve because many liquids do not freeze at the expected. steam above 100°c could be steadily cooled down to 100°c, at which point it would condense to liquid water.. Cooling Curve Of Water Problem.
From www.researchgate.net
Experimental time/temperature cooling curves of water as quenchant. Download Scientific Diagram Cooling Curve Of Water Problem From the phase diagram for water, determine the state of water at:. For cooling curves, we start with the gas phase and remove heat energy. a cooling curve of a substance is a graph of the variation of the temperature with time as it is allowed to cool. 3 shows a heating curve, a plot of temperature versus heating. Cooling Curve Of Water Problem.
From exoqtwrzc.blob.core.windows.net
Heat And Cool Down Faster Than Water at Lillian Roosevelt blog Cooling Curve Of Water Problem The gradient of the cooling curve is related to. As heat is added, the temperature of the ice increases linearly with time. the energy changes that occur during phase changes can be quantified by using a heating or cooling curve. steam above 100°c could be steadily cooled down to 100°c, at which point it would condense to liquid. Cooling Curve Of Water Problem.
From www.expii.com
Heating and Cooling Curves — Overview & Examples Expii Cooling Curve Of Water Problem From the phase diagram for water, determine the state of water at:. As heat is added, the temperature of the ice increases linearly with time. Heating curves and phase changes (problems) page id. The gradient of the cooling curve is related to. for heating curves, we start with a solid and add heat energy. the energy changes that. Cooling Curve Of Water Problem.
From www.researchgate.net
S. Here we show cooling curves for water with added unmixed D 2 O.... Download Scientific Diagram Cooling Curve Of Water Problem the energy changes that occur during phase changes can be quantified by using a heating or cooling curve. for heating curves, we start with a solid and add heat energy. 3 shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. steam above 100°c could be steadily cooled down. Cooling Curve Of Water Problem.
From learningschoolgraciauwb.z4.web.core.windows.net
Heating Curve Of Water Answers Cooling Curve Of Water Problem a cooling curve is not exactly the reverse of the heating curve because many liquids do not freeze at the expected. Heating curves and phase changes (problems) page id. for heating curves, we start with a solid and add heat energy. For cooling curves, we start with the gas phase and remove heat energy. a cooling curve. Cooling Curve Of Water Problem.
From watercoolingsengihi.blogspot.com
Water Cooling Water Cooling Curve Cooling Curve Of Water Problem From the phase diagram for water, determine the state of water at:. steam above 100°c could be steadily cooled down to 100°c, at which point it would condense to liquid water. a cooling curve is not exactly the reverse of the heating curve because many liquids do not freeze at the expected. The sample is initially ice at. Cooling Curve Of Water Problem.
From printablelibagnames.z13.web.core.windows.net
Heating Curve Of Water Explained Cooling Curve Of Water Problem The water could then be. the energy changes that occur during phase changes can be quantified by using a heating or cooling curve. From the phase diagram for water, determine the state of water at:. steam above 100°c could be steadily cooled down to 100°c, at which point it would condense to liquid water. The gradient of the. Cooling Curve Of Water Problem.
From www.coursehero.com
[Solved] Draw a cooling graph of water as it passes from 20°C to 20°C.... Course Hero Cooling Curve Of Water Problem The sample is initially ice at 1 atm and −23°c; 3 shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. Heating curves and phase changes (problems) page id. steam above 100°c could be steadily cooled down to 100°c, at which point it would condense to liquid water. From the phase. Cooling Curve Of Water Problem.
From watercoolingsengihi.blogspot.com
Water Cooling Water Cooling Curve Cooling Curve Of Water Problem For cooling curves, we start with the gas phase and remove heat energy. steam above 100°c could be steadily cooled down to 100°c, at which point it would condense to liquid water. From the phase diagram for water, determine the state of water at:. As heat is added, the temperature of the ice increases linearly with time. a. Cooling Curve Of Water Problem.
From www.researchgate.net
Typical cooling curves of water and aqueous solutions. Download Scientific Diagram Cooling Curve Of Water Problem Heating curves and phase changes (problems) page id. For cooling curves, we start with the gas phase and remove heat energy. a cooling curve of a substance is a graph of the variation of the temperature with time as it is allowed to cool. From the phase diagram for water, determine the state of water at:. a cooling. Cooling Curve Of Water Problem.
From classmediasouconservant.z13.web.core.windows.net
Worksheet Heating Curve Of Water Cooling Curve Of Water Problem a cooling curve is not exactly the reverse of the heating curve because many liquids do not freeze at the expected. The gradient of the cooling curve is related to. for heating curves, we start with a solid and add heat energy. From the phase diagram for water, determine the state of water at:. The water could then. Cooling Curve Of Water Problem.
From youtube.com
Cooling Curves for a LiquidSolid System YouTube Cooling Curve Of Water Problem The sample is initially ice at 1 atm and −23°c; a cooling curve of a substance is a graph of the variation of the temperature with time as it is allowed to cool. The gradient of the cooling curve is related to. The water could then be. Heating curves and phase changes (problems) page id. As heat is added,. Cooling Curve Of Water Problem.
From www.ck12.org
Heating and Cooling Curves CK12 Foundation Cooling Curve Of Water Problem The gradient of the cooling curve is related to. a cooling curve of a substance is a graph of the variation of the temperature with time as it is allowed to cool. From the phase diagram for water, determine the state of water at:. For cooling curves, we start with the gas phase and remove heat energy. The water. Cooling Curve Of Water Problem.
From quizzmediakrueger.z13.web.core.windows.net
Worksheets Heating Curve Of Water Cooling Curve Of Water Problem Heating curves and phase changes (problems) page id. From the phase diagram for water, determine the state of water at:. 3 shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. For cooling curves, we start with the gas phase and remove heat energy. a cooling curve is not exactly the. Cooling Curve Of Water Problem.
From solvedlib.com
Identify cooling the physical curve of water shown O… SolvedLib Cooling Curve Of Water Problem The water could then be. The gradient of the cooling curve is related to. for heating curves, we start with a solid and add heat energy. As heat is added, the temperature of the ice increases linearly with time. 3 shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. . Cooling Curve Of Water Problem.
From www.chegg.com
Solved Heating and Cooling Curve of Water 1. Give the names Cooling Curve Of Water Problem As heat is added, the temperature of the ice increases linearly with time. steam above 100°c could be steadily cooled down to 100°c, at which point it would condense to liquid water. The water could then be. the energy changes that occur during phase changes can be quantified by using a heating or cooling curve. The gradient of. Cooling Curve Of Water Problem.
From www.researchgate.net
Cooling curves of water /PEG (SiCWC). Download Scientific Diagram Cooling Curve Of Water Problem From the phase diagram for water, determine the state of water at:. The sample is initially ice at 1 atm and −23°c; the energy changes that occur during phase changes can be quantified by using a heating or cooling curve. for heating curves, we start with a solid and add heat energy. As heat is added, the temperature. Cooling Curve Of Water Problem.
From www.gauthmath.com
The figure below shows the heating curve of water with four labeled sections on the curve [algebra] Cooling Curve Of Water Problem for heating curves, we start with a solid and add heat energy. The sample is initially ice at 1 atm and −23°c; As heat is added, the temperature of the ice increases linearly with time. The gradient of the cooling curve is related to. a cooling curve of a substance is a graph of the variation of the. Cooling Curve Of Water Problem.
From blog.doingsciencetostuff.com
Cooling Curve For Water Doing Science To Stuff Cooling Curve Of Water Problem a cooling curve of a substance is a graph of the variation of the temperature with time as it is allowed to cool. As heat is added, the temperature of the ice increases linearly with time. the energy changes that occur during phase changes can be quantified by using a heating or cooling curve. From the phase diagram. Cooling Curve Of Water Problem.
From www.chegg.com
Solved Use the following cooling curve of water to answer Cooling Curve Of Water Problem Heating curves and phase changes (problems) page id. a cooling curve is not exactly the reverse of the heating curve because many liquids do not freeze at the expected. The gradient of the cooling curve is related to. The water could then be. the energy changes that occur during phase changes can be quantified by using a heating. Cooling Curve Of Water Problem.
From spmchemistry.blog.onlinetuition.com.my
Cooling Curve SPM Chemistry Cooling Curve Of Water Problem a cooling curve of a substance is a graph of the variation of the temperature with time as it is allowed to cool. a cooling curve is not exactly the reverse of the heating curve because many liquids do not freeze at the expected. As heat is added, the temperature of the ice increases linearly with time. The. Cooling Curve Of Water Problem.
From slidetodoc.com
CALCULATING ENERGY CHANGES HEATING CURVE OF WATER COOLING Cooling Curve Of Water Problem a cooling curve of a substance is a graph of the variation of the temperature with time as it is allowed to cool. steam above 100°c could be steadily cooled down to 100°c, at which point it would condense to liquid water. As heat is added, the temperature of the ice increases linearly with time. 3 shows a. Cooling Curve Of Water Problem.
From www.researchgate.net
S. The reproducibility of the cooling curve for water with no heavy... Download Scientific Diagram Cooling Curve Of Water Problem From the phase diagram for water, determine the state of water at:. a cooling curve of a substance is a graph of the variation of the temperature with time as it is allowed to cool. For cooling curves, we start with the gas phase and remove heat energy. for heating curves, we start with a solid and add. Cooling Curve Of Water Problem.
From quizzcampusduran101.z13.web.core.windows.net
Heating Curve Of Water Worksheet Cooling Curve Of Water Problem the energy changes that occur during phase changes can be quantified by using a heating or cooling curve. a cooling curve of a substance is a graph of the variation of the temperature with time as it is allowed to cool. Heating curves and phase changes (problems) page id. The water could then be. 3 shows a heating. Cooling Curve Of Water Problem.
From learningschoolgraciauwb.z4.web.core.windows.net
Heating Curve Of Water Cooling Curve Of Water Problem the energy changes that occur during phase changes can be quantified by using a heating or cooling curve. The sample is initially ice at 1 atm and −23°c; For cooling curves, we start with the gas phase and remove heat energy. for heating curves, we start with a solid and add heat energy. Heating curves and phase changes. Cooling Curve Of Water Problem.
From dxouiuygg.blob.core.windows.net
How To Cool Water Below Freezing at Thomas Pineiro blog Cooling Curve Of Water Problem The sample is initially ice at 1 atm and −23°c; As heat is added, the temperature of the ice increases linearly with time. a cooling curve is not exactly the reverse of the heating curve because many liquids do not freeze at the expected. The gradient of the cooling curve is related to. From the phase diagram for water,. Cooling Curve Of Water Problem.
From slidetodoc.com
CALCULATING ENERGY CHANGES HEATING CURVE OF WATER COOLING Cooling Curve Of Water Problem 3 shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. The gradient of the cooling curve is related to. the energy changes that occur during phase changes can be quantified by using a heating or cooling curve. The water could then be. As heat is added, the temperature of the. Cooling Curve Of Water Problem.
From www.youtube.com
Heating and Cooling Curve for Water YouTube Cooling Curve Of Water Problem the energy changes that occur during phase changes can be quantified by using a heating or cooling curve. for heating curves, we start with a solid and add heat energy. steam above 100°c could be steadily cooled down to 100°c, at which point it would condense to liquid water. For cooling curves, we start with the gas. Cooling Curve Of Water Problem.
From www.youtube.com
Heating Curve and Cooling Curve of Water Enthalpy of Fusion & Vaporization YouTube Cooling Curve Of Water Problem a cooling curve is not exactly the reverse of the heating curve because many liquids do not freeze at the expected. 3 shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. The gradient of the cooling curve is related to. As heat is added, the temperature of the ice increases. Cooling Curve Of Water Problem.
From www.docsity.com
THE HEATING CURVE OF WATER Slides Chemistry Docsity Cooling Curve Of Water Problem a cooling curve is not exactly the reverse of the heating curve because many liquids do not freeze at the expected. Heating curves and phase changes (problems) page id. a cooling curve of a substance is a graph of the variation of the temperature with time as it is allowed to cool. 3 shows a heating curve, a. Cooling Curve Of Water Problem.