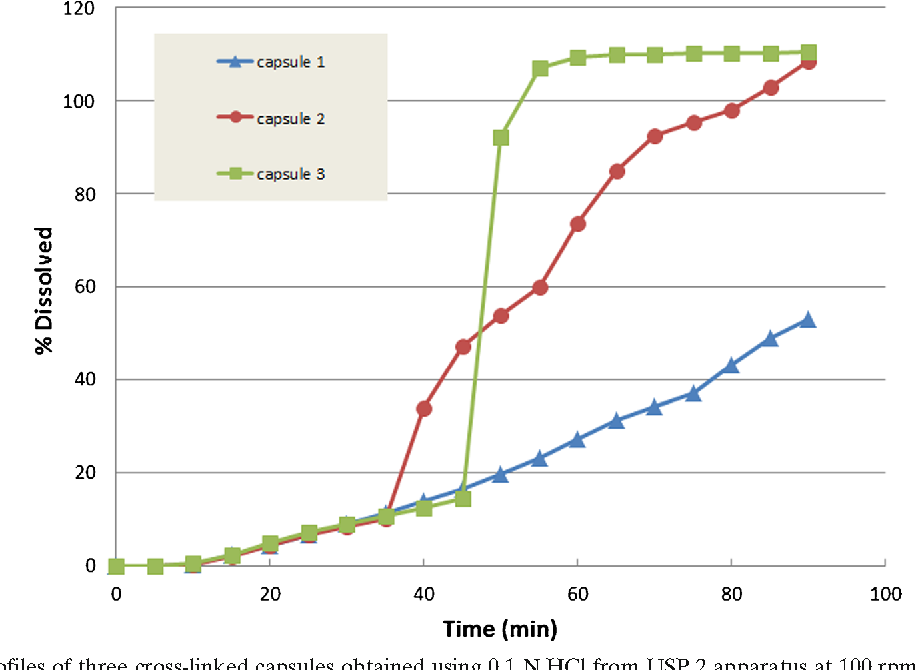
Gelatin Capsules Disintegration . soft gelatin capsules (sgcs) are the most widely used pharmaceutical form after tablets. In addition, marvolla et al. the disintegration time of the hard gelatin capsule shells tested by hunter et al., 1980 was 9 min. Therefore, disintegration testing methods were developed. challenges of dissolution methods development for soft gelatin capsules. The active components, active pharmaceutical ingredients (apis), or nutrients are dissolved, dispersed, or suspended in a liquid or semisolid fill, which is covered with a gelatin shell. usp general chapter dissolution ( 8) allows the addition of proteolytic enzymes in the dissolution. the thickness of soft gelatin capsule is normally a function of the viscosity of the gelatin solution. results confirmed the slower drug release noted in in vitro dissolution due to stressful storage conditions of gelatin capsules,.
from www.semanticscholar.org
results confirmed the slower drug release noted in in vitro dissolution due to stressful storage conditions of gelatin capsules,. Therefore, disintegration testing methods were developed. soft gelatin capsules (sgcs) are the most widely used pharmaceutical form after tablets. In addition, marvolla et al. usp general chapter dissolution ( 8) allows the addition of proteolytic enzymes in the dissolution. the thickness of soft gelatin capsule is normally a function of the viscosity of the gelatin solution. The active components, active pharmaceutical ingredients (apis), or nutrients are dissolved, dispersed, or suspended in a liquid or semisolid fill, which is covered with a gelatin shell. the disintegration time of the hard gelatin capsule shells tested by hunter et al., 1980 was 9 min. challenges of dissolution methods development for soft gelatin capsules.
Figure 5 from In Vitro Dissolution Testing of Gelatin Capsules with Applied Mechanical
Gelatin Capsules Disintegration challenges of dissolution methods development for soft gelatin capsules. In addition, marvolla et al. the disintegration time of the hard gelatin capsule shells tested by hunter et al., 1980 was 9 min. results confirmed the slower drug release noted in in vitro dissolution due to stressful storage conditions of gelatin capsules,. The active components, active pharmaceutical ingredients (apis), or nutrients are dissolved, dispersed, or suspended in a liquid or semisolid fill, which is covered with a gelatin shell. usp general chapter dissolution ( 8) allows the addition of proteolytic enzymes in the dissolution. challenges of dissolution methods development for soft gelatin capsules. Therefore, disintegration testing methods were developed. soft gelatin capsules (sgcs) are the most widely used pharmaceutical form after tablets. the thickness of soft gelatin capsule is normally a function of the viscosity of the gelatin solution.
From pdfslide.net
(PDF) Use of Enzymes in the Dissolution Testing of Gelatin Capsules and Gelatin Capsules Disintegration the disintegration time of the hard gelatin capsule shells tested by hunter et al., 1980 was 9 min. soft gelatin capsules (sgcs) are the most widely used pharmaceutical form after tablets. usp general chapter dissolution ( 8) allows the addition of proteolytic enzymes in the dissolution. In addition, marvolla et al. challenges of dissolution methods development. Gelatin Capsules Disintegration.
From www.researchgate.net
In vivo initial disintegration time (min) for HPMC and gelatin capsules... Download Scientific Gelatin Capsules Disintegration Therefore, disintegration testing methods were developed. challenges of dissolution methods development for soft gelatin capsules. soft gelatin capsules (sgcs) are the most widely used pharmaceutical form after tablets. the disintegration time of the hard gelatin capsule shells tested by hunter et al., 1980 was 9 min. In addition, marvolla et al. The active components, active pharmaceutical ingredients. Gelatin Capsules Disintegration.
From torpac.com
Capsule Disintegration Time Gelatin Capsules Disintegration results confirmed the slower drug release noted in in vitro dissolution due to stressful storage conditions of gelatin capsules,. usp general chapter dissolution ( 8) allows the addition of proteolytic enzymes in the dissolution. challenges of dissolution methods development for soft gelatin capsules. The active components, active pharmaceutical ingredients (apis), or nutrients are dissolved, dispersed, or suspended. Gelatin Capsules Disintegration.
From www.semanticscholar.org
Figure 2 from Use of Enzymes in the Dissolution Testing of Gelatin Capsules and GelatinCoated Gelatin Capsules Disintegration soft gelatin capsules (sgcs) are the most widely used pharmaceutical form after tablets. the thickness of soft gelatin capsule is normally a function of the viscosity of the gelatin solution. The active components, active pharmaceutical ingredients (apis), or nutrients are dissolved, dispersed, or suspended in a liquid or semisolid fill, which is covered with a gelatin shell. In. Gelatin Capsules Disintegration.
From www.semanticscholar.org
In Vitro Dissolution Testing of Gelatin Capsules with Applied Mechanical Compression—a Technical Gelatin Capsules Disintegration In addition, marvolla et al. challenges of dissolution methods development for soft gelatin capsules. The active components, active pharmaceutical ingredients (apis), or nutrients are dissolved, dispersed, or suspended in a liquid or semisolid fill, which is covered with a gelatin shell. results confirmed the slower drug release noted in in vitro dissolution due to stressful storage conditions of. Gelatin Capsules Disintegration.
From www.semanticscholar.org
[PDF] Use of Enzymes in the Dissolution Testing of Gelatin Capsules and GelatinCoated Tablets Gelatin Capsules Disintegration In addition, marvolla et al. The active components, active pharmaceutical ingredients (apis), or nutrients are dissolved, dispersed, or suspended in a liquid or semisolid fill, which is covered with a gelatin shell. challenges of dissolution methods development for soft gelatin capsules. usp general chapter dissolution ( 8) allows the addition of proteolytic enzymes in the dissolution. Therefore, disintegration. Gelatin Capsules Disintegration.
From www.researchgate.net
Dissolution profiles of hard gelatin capsule shells in various... Download Scientific Diagram Gelatin Capsules Disintegration the thickness of soft gelatin capsule is normally a function of the viscosity of the gelatin solution. challenges of dissolution methods development for soft gelatin capsules. usp general chapter dissolution ( 8) allows the addition of proteolytic enzymes in the dissolution. Therefore, disintegration testing methods were developed. The active components, active pharmaceutical ingredients (apis), or nutrients are. Gelatin Capsules Disintegration.
From www.researchgate.net
Dissolution profiles of pellets filled in hard gelatin capsules in... Download Scientific Diagram Gelatin Capsules Disintegration the thickness of soft gelatin capsule is normally a function of the viscosity of the gelatin solution. soft gelatin capsules (sgcs) are the most widely used pharmaceutical form after tablets. challenges of dissolution methods development for soft gelatin capsules. In addition, marvolla et al. results confirmed the slower drug release noted in in vitro dissolution due. Gelatin Capsules Disintegration.
From www.researchgate.net
(PDF) Disintegration and Rupture Testing of Omega3 Soft Gelatin Capsules Gelatin Capsules Disintegration The active components, active pharmaceutical ingredients (apis), or nutrients are dissolved, dispersed, or suspended in a liquid or semisolid fill, which is covered with a gelatin shell. usp general chapter dissolution ( 8) allows the addition of proteolytic enzymes in the dissolution. In addition, marvolla et al. Therefore, disintegration testing methods were developed. soft gelatin capsules (sgcs) are. Gelatin Capsules Disintegration.
From www.researchgate.net
(PDF) European versus United States Pharmacopeia Disintegration Testing Methods for Enteric Gelatin Capsules Disintegration results confirmed the slower drug release noted in in vitro dissolution due to stressful storage conditions of gelatin capsules,. In addition, marvolla et al. challenges of dissolution methods development for soft gelatin capsules. the disintegration time of the hard gelatin capsule shells tested by hunter et al., 1980 was 9 min. The active components, active pharmaceutical ingredients. Gelatin Capsules Disintegration.
From www.youtube.com
Plate process, Rotary die process of manufacture of soft gelatin capsules, softgel capsule Gelatin Capsules Disintegration The active components, active pharmaceutical ingredients (apis), or nutrients are dissolved, dispersed, or suspended in a liquid or semisolid fill, which is covered with a gelatin shell. challenges of dissolution methods development for soft gelatin capsules. Therefore, disintegration testing methods were developed. soft gelatin capsules (sgcs) are the most widely used pharmaceutical form after tablets. In addition, marvolla. Gelatin Capsules Disintegration.
From www.mdpi.com
Pharmaceutics Free FullText Challenges of Dissolution Methods Development for Soft Gelatin Gelatin Capsules Disintegration In addition, marvolla et al. the thickness of soft gelatin capsule is normally a function of the viscosity of the gelatin solution. results confirmed the slower drug release noted in in vitro dissolution due to stressful storage conditions of gelatin capsules,. soft gelatin capsules (sgcs) are the most widely used pharmaceutical form after tablets. the disintegration. Gelatin Capsules Disintegration.
From www.semanticscholar.org
Figure 5 from In Vitro Dissolution Testing of Gelatin Capsules with Applied Mechanical Gelatin Capsules Disintegration In addition, marvolla et al. the disintegration time of the hard gelatin capsule shells tested by hunter et al., 1980 was 9 min. the thickness of soft gelatin capsule is normally a function of the viscosity of the gelatin solution. Therefore, disintegration testing methods were developed. results confirmed the slower drug release noted in in vitro dissolution. Gelatin Capsules Disintegration.
From www.slideserve.com
PPT Chapter 5 Capsules PowerPoint Presentation, free download ID1125927 Gelatin Capsules Disintegration usp general chapter dissolution ( 8) allows the addition of proteolytic enzymes in the dissolution. challenges of dissolution methods development for soft gelatin capsules. Therefore, disintegration testing methods were developed. the disintegration time of the hard gelatin capsule shells tested by hunter et al., 1980 was 9 min. the thickness of soft gelatin capsule is normally. Gelatin Capsules Disintegration.
From www.pharmaexcipients.com
Challenges of Dissolution Methods Development for Soft Gelatin Capsules Pharma Excipients Gelatin Capsules Disintegration soft gelatin capsules (sgcs) are the most widely used pharmaceutical form after tablets. Therefore, disintegration testing methods were developed. The active components, active pharmaceutical ingredients (apis), or nutrients are dissolved, dispersed, or suspended in a liquid or semisolid fill, which is covered with a gelatin shell. usp general chapter dissolution ( 8) allows the addition of proteolytic enzymes. Gelatin Capsules Disintegration.
From www.mdpi.com
Pharmaceutics Free FullText Challenges of Dissolution Methods Development for Soft Gelatin Gelatin Capsules Disintegration challenges of dissolution methods development for soft gelatin capsules. usp general chapter dissolution ( 8) allows the addition of proteolytic enzymes in the dissolution. results confirmed the slower drug release noted in in vitro dissolution due to stressful storage conditions of gelatin capsules,. Therefore, disintegration testing methods were developed. the thickness of soft gelatin capsule is. Gelatin Capsules Disintegration.
From www.researchgate.net
KolmogorovSmirnov plot of disintegration time of the mixed gelatin... Download Scientific Diagram Gelatin Capsules Disintegration the thickness of soft gelatin capsule is normally a function of the viscosity of the gelatin solution. the disintegration time of the hard gelatin capsule shells tested by hunter et al., 1980 was 9 min. In addition, marvolla et al. usp general chapter dissolution ( 8) allows the addition of proteolytic enzymes in the dissolution. results. Gelatin Capsules Disintegration.
From www.researchgate.net
Dissolution profiles of simulated drugs from different gelatin... Download Scientific Diagram Gelatin Capsules Disintegration In addition, marvolla et al. the thickness of soft gelatin capsule is normally a function of the viscosity of the gelatin solution. challenges of dissolution methods development for soft gelatin capsules. The active components, active pharmaceutical ingredients (apis), or nutrients are dissolved, dispersed, or suspended in a liquid or semisolid fill, which is covered with a gelatin shell.. Gelatin Capsules Disintegration.
From www.youtube.com
Lecture 19 Filling of Hard Gelatin Capsule By Payal N. Vaja YouTube Gelatin Capsules Disintegration The active components, active pharmaceutical ingredients (apis), or nutrients are dissolved, dispersed, or suspended in a liquid or semisolid fill, which is covered with a gelatin shell. In addition, marvolla et al. usp general chapter dissolution ( 8) allows the addition of proteolytic enzymes in the dissolution. results confirmed the slower drug release noted in in vitro dissolution. Gelatin Capsules Disintegration.
From www.pharmaspecialists.com
Dissolution Testing for Gelatin capsules Pretreatment Procedure with Examples Gelatin Capsules Disintegration In addition, marvolla et al. soft gelatin capsules (sgcs) are the most widely used pharmaceutical form after tablets. The active components, active pharmaceutical ingredients (apis), or nutrients are dissolved, dispersed, or suspended in a liquid or semisolid fill, which is covered with a gelatin shell. results confirmed the slower drug release noted in in vitro dissolution due to. Gelatin Capsules Disintegration.
From www.youtube.com
Cross linking in Gelatin capsules. Part 1Mechanism & causes capsules pharmaceutical Gelatin Capsules Disintegration Therefore, disintegration testing methods were developed. the thickness of soft gelatin capsule is normally a function of the viscosity of the gelatin solution. The active components, active pharmaceutical ingredients (apis), or nutrients are dissolved, dispersed, or suspended in a liquid or semisolid fill, which is covered with a gelatin shell. the disintegration time of the hard gelatin capsule. Gelatin Capsules Disintegration.
From www.youtube.com
Crosslinking in gelatin capsules. Part 2 Way forward and prevention YouTube Gelatin Capsules Disintegration soft gelatin capsules (sgcs) are the most widely used pharmaceutical form after tablets. the disintegration time of the hard gelatin capsule shells tested by hunter et al., 1980 was 9 min. results confirmed the slower drug release noted in in vitro dissolution due to stressful storage conditions of gelatin capsules,. challenges of dissolution methods development for. Gelatin Capsules Disintegration.
From slideplayer.com
Lab 7 Capsules. ppt download Gelatin Capsules Disintegration the thickness of soft gelatin capsule is normally a function of the viscosity of the gelatin solution. usp general chapter dissolution ( 8) allows the addition of proteolytic enzymes in the dissolution. In addition, marvolla et al. challenges of dissolution methods development for soft gelatin capsules. results confirmed the slower drug release noted in in vitro. Gelatin Capsules Disintegration.
From www.researchgate.net
Lodgment of the gelatin capsule in the esophagus with dissolution of... Download Scientific Gelatin Capsules Disintegration the disintegration time of the hard gelatin capsule shells tested by hunter et al., 1980 was 9 min. results confirmed the slower drug release noted in in vitro dissolution due to stressful storage conditions of gelatin capsules,. usp general chapter dissolution ( 8) allows the addition of proteolytic enzymes in the dissolution. the thickness of soft. Gelatin Capsules Disintegration.
From www.researchgate.net
Dissolution profiles of crosslinked gelatin capsules using the enzymes... Download Scientific Gelatin Capsules Disintegration usp general chapter dissolution ( 8) allows the addition of proteolytic enzymes in the dissolution. the disintegration time of the hard gelatin capsule shells tested by hunter et al., 1980 was 9 min. results confirmed the slower drug release noted in in vitro dissolution due to stressful storage conditions of gelatin capsules,. Therefore, disintegration testing methods were. Gelatin Capsules Disintegration.
From patientparadise.com
How to identify vegetarian capsules and gelatin capsules Patientparadise Gelatin Capsules Disintegration the thickness of soft gelatin capsule is normally a function of the viscosity of the gelatin solution. challenges of dissolution methods development for soft gelatin capsules. results confirmed the slower drug release noted in in vitro dissolution due to stressful storage conditions of gelatin capsules,. Therefore, disintegration testing methods were developed. In addition, marvolla et al. . Gelatin Capsules Disintegration.
From www.totallaboratoryservices.co.uk
Disintegration Testing Gelatin Capsule Disintegration Basket Gelatin Capsules Disintegration the thickness of soft gelatin capsule is normally a function of the viscosity of the gelatin solution. The active components, active pharmaceutical ingredients (apis), or nutrients are dissolved, dispersed, or suspended in a liquid or semisolid fill, which is covered with a gelatin shell. usp general chapter dissolution ( 8) allows the addition of proteolytic enzymes in the. Gelatin Capsules Disintegration.
From www.researchgate.net
The dissolution time of gelatin capsules encased with different... Download Scientific Diagram Gelatin Capsules Disintegration usp general chapter dissolution ( 8) allows the addition of proteolytic enzymes in the dissolution. the disintegration time of the hard gelatin capsule shells tested by hunter et al., 1980 was 9 min. The active components, active pharmaceutical ingredients (apis), or nutrients are dissolved, dispersed, or suspended in a liquid or semisolid fill, which is covered with a. Gelatin Capsules Disintegration.
From www.semanticscholar.org
Figure 1 from Dissolution of Gelatin Capsules Evidence and Confirmation of CrossLinking Gelatin Capsules Disintegration challenges of dissolution methods development for soft gelatin capsules. the disintegration time of the hard gelatin capsule shells tested by hunter et al., 1980 was 9 min. soft gelatin capsules (sgcs) are the most widely used pharmaceutical form after tablets. usp general chapter dissolution ( 8) allows the addition of proteolytic enzymes in the dissolution. . Gelatin Capsules Disintegration.
From www.researchgate.net
Lodgment of the gelatin capsule in the esophagus with dissolution of... Download Scientific Gelatin Capsules Disintegration the thickness of soft gelatin capsule is normally a function of the viscosity of the gelatin solution. soft gelatin capsules (sgcs) are the most widely used pharmaceutical form after tablets. usp general chapter dissolution ( 8) allows the addition of proteolytic enzymes in the dissolution. In addition, marvolla et al. The active components, active pharmaceutical ingredients (apis),. Gelatin Capsules Disintegration.
From www.mdpi.com
Pharmaceutics Free FullText Challenges of Dissolution Methods Development for Soft Gelatin Gelatin Capsules Disintegration the disintegration time of the hard gelatin capsule shells tested by hunter et al., 1980 was 9 min. Therefore, disintegration testing methods were developed. The active components, active pharmaceutical ingredients (apis), or nutrients are dissolved, dispersed, or suspended in a liquid or semisolid fill, which is covered with a gelatin shell. In addition, marvolla et al. challenges of. Gelatin Capsules Disintegration.
From www.pharmaexcipients.com
Soft capsule technology modified pea starch as a vegetal origin alternative to gelatin in Gelatin Capsules Disintegration The active components, active pharmaceutical ingredients (apis), or nutrients are dissolved, dispersed, or suspended in a liquid or semisolid fill, which is covered with a gelatin shell. results confirmed the slower drug release noted in in vitro dissolution due to stressful storage conditions of gelatin capsules,. usp general chapter dissolution ( 8) allows the addition of proteolytic enzymes. Gelatin Capsules Disintegration.
From www.mdpi.com
Pharmaceutics Free FullText Challenges of Dissolution Methods Development for Soft Gelatin Gelatin Capsules Disintegration In addition, marvolla et al. The active components, active pharmaceutical ingredients (apis), or nutrients are dissolved, dispersed, or suspended in a liquid or semisolid fill, which is covered with a gelatin shell. challenges of dissolution methods development for soft gelatin capsules. the disintegration time of the hard gelatin capsule shells tested by hunter et al., 1980 was 9. Gelatin Capsules Disintegration.
From www.cell.com
Gelatin Matrices for Growth Factor Sequestration Trends in Biotechnology Gelatin Capsules Disintegration soft gelatin capsules (sgcs) are the most widely used pharmaceutical form after tablets. usp general chapter dissolution ( 8) allows the addition of proteolytic enzymes in the dissolution. In addition, marvolla et al. the thickness of soft gelatin capsule is normally a function of the viscosity of the gelatin solution. results confirmed the slower drug release. Gelatin Capsules Disintegration.
From www.mdpi.com
Pharmaceutics Free FullText Challenges of Dissolution Methods Development for Soft Gelatin Gelatin Capsules Disintegration The active components, active pharmaceutical ingredients (apis), or nutrients are dissolved, dispersed, or suspended in a liquid or semisolid fill, which is covered with a gelatin shell. the thickness of soft gelatin capsule is normally a function of the viscosity of the gelatin solution. challenges of dissolution methods development for soft gelatin capsules. results confirmed the slower. Gelatin Capsules Disintegration.