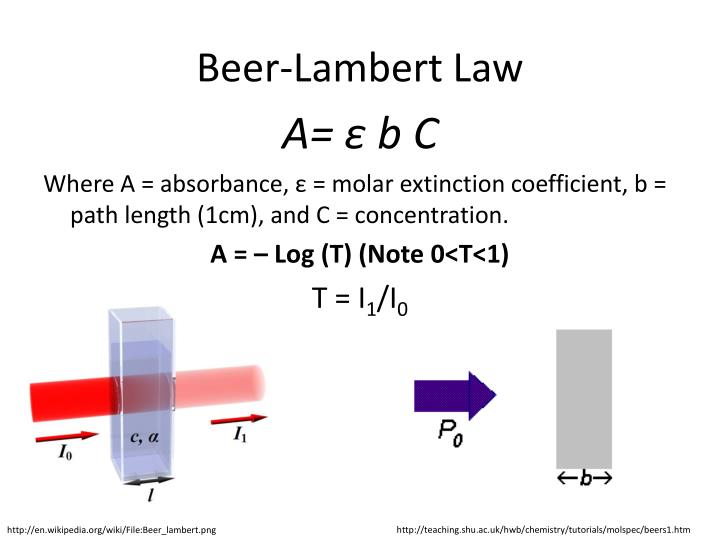
Beer Lambert Law Ap Chemistry Questions . The actual solution concentration must have been lower than 0.150m, because the reported absorbance value is too small based on the. Study with quizlet and memorize flashcards containing terms like beers law equation, color wavelengths, absorbance and more. What will be the units of ∈, the specific. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Answer the following questions regarding light and its interactions with molecules, atoms, and ions. [1] the absorbance is a dimensionless quantity, that is, a quantity without units. (a) the longest wavelength of.
from fyooddpjn.blob.core.windows.net
What will be the units of ∈, the specific. The actual solution concentration must have been lower than 0.150m, because the reported absorbance value is too small based on the. Study with quizlet and memorize flashcards containing terms like beers law equation, color wavelengths, absorbance and more. Answer the following questions regarding light and its interactions with molecules, atoms, and ions. (a) the longest wavelength of. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. [1] the absorbance is a dimensionless quantity, that is, a quantity without units.
Beer Lambert Law Enzyme Activity at Russell Bingaman blog
Beer Lambert Law Ap Chemistry Questions Answer the following questions regarding light and its interactions with molecules, atoms, and ions. Answer the following questions regarding light and its interactions with molecules, atoms, and ions. (a) the longest wavelength of. Study with quizlet and memorize flashcards containing terms like beers law equation, color wavelengths, absorbance and more. The actual solution concentration must have been lower than 0.150m, because the reported absorbance value is too small based on the. [1] the absorbance is a dimensionless quantity, that is, a quantity without units. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. What will be the units of ∈, the specific.
From www.studypool.com
SOLUTION Beer Lambert Law Question and Answers Studypool Beer Lambert Law Ap Chemistry Questions The actual solution concentration must have been lower than 0.150m, because the reported absorbance value is too small based on the. What will be the units of ∈, the specific. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Study with quizlet and memorize flashcards containing terms like beers law equation, color wavelengths,. Beer Lambert Law Ap Chemistry Questions.
From www.geeksforgeeks.org
BeerLambert Law Statement, Formula, Equation & Derivation Beer Lambert Law Ap Chemistry Questions (a) the longest wavelength of. [1] the absorbance is a dimensionless quantity, that is, a quantity without units. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Study with quizlet and memorize flashcards containing terms like beers law equation, color wavelengths, absorbance and more. What will be the units of ∈, the specific.. Beer Lambert Law Ap Chemistry Questions.
From www.youtube.com
Absorbance Transmittance Numerical Practice problem on Lambert Beer Beer Lambert Law Ap Chemistry Questions Answer the following questions regarding light and its interactions with molecules, atoms, and ions. (a) the longest wavelength of. The actual solution concentration must have been lower than 0.150m, because the reported absorbance value is too small based on the. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Study with quizlet and. Beer Lambert Law Ap Chemistry Questions.
From www.youtube.com
Chem 1 Lab 4 terms 1 BeerLambert law equation YouTube Beer Lambert Law Ap Chemistry Questions What will be the units of ∈, the specific. (a) the longest wavelength of. The actual solution concentration must have been lower than 0.150m, because the reported absorbance value is too small based on the. Study with quizlet and memorize flashcards containing terms like beers law equation, color wavelengths, absorbance and more. Answer the following questions regarding light and its. Beer Lambert Law Ap Chemistry Questions.
From app.formative.com
Beer's Law Simulation for AP Chemistry Carson Dobrin Library Beer Lambert Law Ap Chemistry Questions What will be the units of ∈, the specific. The actual solution concentration must have been lower than 0.150m, because the reported absorbance value is too small based on the. (a) the longest wavelength of. [1] the absorbance is a dimensionless quantity, that is, a quantity without units. In most experiments, molar absorptivity (ε) and the length (b) are constant,. Beer Lambert Law Ap Chemistry Questions.
From www.youtube.com
Worked example Calculating concentration using the BeerLambert law Beer Lambert Law Ap Chemistry Questions (a) the longest wavelength of. What will be the units of ∈, the specific. The actual solution concentration must have been lower than 0.150m, because the reported absorbance value is too small based on the. Answer the following questions regarding light and its interactions with molecules, atoms, and ions. [1] the absorbance is a dimensionless quantity, that is, a quantity. Beer Lambert Law Ap Chemistry Questions.
From chemistrytalk.org
BeerLambert Law ChemTalk Beer Lambert Law Ap Chemistry Questions In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. [1] the absorbance is a dimensionless quantity, that is, a quantity without units. (a) the longest wavelength of. Study with quizlet and memorize flashcards containing terms like beers law equation, color wavelengths, absorbance and more. The actual solution concentration must have been lower than. Beer Lambert Law Ap Chemistry Questions.
From scienceinfo.com
BeerLambert Law Statement, Derivation, Applications, Limitations Beer Lambert Law Ap Chemistry Questions (a) the longest wavelength of. [1] the absorbance is a dimensionless quantity, that is, a quantity without units. The actual solution concentration must have been lower than 0.150m, because the reported absorbance value is too small based on the. What will be the units of ∈, the specific. Study with quizlet and memorize flashcards containing terms like beers law equation,. Beer Lambert Law Ap Chemistry Questions.
From www.chegg.com
Solved um 7. CHEM 1110 Lab. Exercise The BeerLambert Law Beer Lambert Law Ap Chemistry Questions Answer the following questions regarding light and its interactions with molecules, atoms, and ions. Study with quizlet and memorize flashcards containing terms like beers law equation, color wavelengths, absorbance and more. The actual solution concentration must have been lower than 0.150m, because the reported absorbance value is too small based on the. In most experiments, molar absorptivity (ε) and the. Beer Lambert Law Ap Chemistry Questions.
From www.chegg.com
Solved S SUR CHEM 242 Online Lab 3 BeerLambert Law Beer Lambert Law Ap Chemistry Questions Study with quizlet and memorize flashcards containing terms like beers law equation, color wavelengths, absorbance and more. (a) the longest wavelength of. What will be the units of ∈, the specific. Answer the following questions regarding light and its interactions with molecules, atoms, and ions. The actual solution concentration must have been lower than 0.150m, because the reported absorbance value. Beer Lambert Law Ap Chemistry Questions.
From www.youtube.com
Spectrophotometry and the BeerLambert Law AP Chemistry Khan Beer Lambert Law Ap Chemistry Questions The actual solution concentration must have been lower than 0.150m, because the reported absorbance value is too small based on the. [1] the absorbance is a dimensionless quantity, that is, a quantity without units. (a) the longest wavelength of. Study with quizlet and memorize flashcards containing terms like beers law equation, color wavelengths, absorbance and more. In most experiments, molar. Beer Lambert Law Ap Chemistry Questions.
From www.scribd.com
CHEM 242 Online Lab 3 BeerLambert Law Worksheet PDF Beer Lambert Law Ap Chemistry Questions In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Study with quizlet and memorize flashcards containing terms like beers law equation, color wavelengths, absorbance and more. Answer the following questions regarding light and its interactions with molecules, atoms, and ions. [1] the absorbance is a dimensionless quantity, that is, a quantity without units.. Beer Lambert Law Ap Chemistry Questions.
From www.chegg.com
Solved BeerLambert Law Application Example 1 A compound Beer Lambert Law Ap Chemistry Questions What will be the units of ∈, the specific. (a) the longest wavelength of. The actual solution concentration must have been lower than 0.150m, because the reported absorbance value is too small based on the. [1] the absorbance is a dimensionless quantity, that is, a quantity without units. In most experiments, molar absorptivity (ε) and the length (b) are constant,. Beer Lambert Law Ap Chemistry Questions.
From www.pinterest.com
Beer's Law Worksheet 3 pages, 3 multipart problems. Ap chemistry Beer Lambert Law Ap Chemistry Questions What will be the units of ∈, the specific. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Answer the following questions regarding light and its interactions with molecules, atoms, and ions. [1] the absorbance is a dimensionless quantity, that is, a quantity without units. Study with quizlet and memorize flashcards containing terms. Beer Lambert Law Ap Chemistry Questions.
From mungfali.com
UV Spectroscopy Beer Lambert Law Beer Lambert Law Ap Chemistry Questions What will be the units of ∈, the specific. The actual solution concentration must have been lower than 0.150m, because the reported absorbance value is too small based on the. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Answer the following questions regarding light and its interactions with molecules, atoms, and ions.. Beer Lambert Law Ap Chemistry Questions.
From chemistrytalk.org
BeerLambert Law ChemTalk Beer Lambert Law Ap Chemistry Questions The actual solution concentration must have been lower than 0.150m, because the reported absorbance value is too small based on the. (a) the longest wavelength of. [1] the absorbance is a dimensionless quantity, that is, a quantity without units. Answer the following questions regarding light and its interactions with molecules, atoms, and ions. In most experiments, molar absorptivity (ε) and. Beer Lambert Law Ap Chemistry Questions.
From parcoscientific.com
AP Chemistry Beer Lambert Law Classics AP Chemistry Kits Chemistry Beer Lambert Law Ap Chemistry Questions (a) the longest wavelength of. The actual solution concentration must have been lower than 0.150m, because the reported absorbance value is too small based on the. Answer the following questions regarding light and its interactions with molecules, atoms, and ions. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Study with quizlet and. Beer Lambert Law Ap Chemistry Questions.
From calculatorghw.blogspot.com
Beer Lambert Law Calculator CALCULATOR GHW Beer Lambert Law Ap Chemistry Questions (a) the longest wavelength of. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. [1] the absorbance is a dimensionless quantity, that is, a quantity without units. The actual solution concentration must have been lower than 0.150m, because the reported absorbance value is too small based on the. What will be the units. Beer Lambert Law Ap Chemistry Questions.
From fyooddpjn.blob.core.windows.net
Beer Lambert Law Enzyme Activity at Russell Bingaman blog Beer Lambert Law Ap Chemistry Questions The actual solution concentration must have been lower than 0.150m, because the reported absorbance value is too small based on the. Study with quizlet and memorize flashcards containing terms like beers law equation, color wavelengths, absorbance and more. What will be the units of ∈, the specific. Answer the following questions regarding light and its interactions with molecules, atoms, and. Beer Lambert Law Ap Chemistry Questions.
From study.com
How to Find the Path Length Using the BeerLambert Law Chemistry Beer Lambert Law Ap Chemistry Questions (a) the longest wavelength of. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. The actual solution concentration must have been lower than 0.150m, because the reported absorbance value is too small based on the. What will be the units of ∈, the specific. Answer the following questions regarding light and its interactions. Beer Lambert Law Ap Chemistry Questions.
From www.studocu.com
AP Chem Unit 3.13 BeerLambert Law AP Chemistry Fiveable Reading Beer Lambert Law Ap Chemistry Questions (a) the longest wavelength of. The actual solution concentration must have been lower than 0.150m, because the reported absorbance value is too small based on the. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Study with quizlet and memorize flashcards containing terms like beers law equation, color wavelengths, absorbance and more. What. Beer Lambert Law Ap Chemistry Questions.
From www.youtube.com
AP Chemistry Investigation 1 BeerLambert Law. YouTube Beer Lambert Law Ap Chemistry Questions Answer the following questions regarding light and its interactions with molecules, atoms, and ions. Study with quizlet and memorize flashcards containing terms like beers law equation, color wavelengths, absorbance and more. What will be the units of ∈, the specific. [1] the absorbance is a dimensionless quantity, that is, a quantity without units. (a) the longest wavelength of. The actual. Beer Lambert Law Ap Chemistry Questions.
From www.studocu.com
Biochemistry Few Questions and answers based on BeerLambert Law and Beer Lambert Law Ap Chemistry Questions In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. (a) the longest wavelength of. What will be the units of ∈, the specific. The actual solution concentration must have been lower than 0.150m, because the reported absorbance value is too small based on the. [1] the absorbance is a dimensionless quantity, that is,. Beer Lambert Law Ap Chemistry Questions.
From www.studypool.com
SOLUTION 2 beer lambert law complete Studypool Beer Lambert Law Ap Chemistry Questions Answer the following questions regarding light and its interactions with molecules, atoms, and ions. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. [1] the absorbance is a dimensionless quantity, that is, a quantity without units. The actual solution concentration must have been lower than 0.150m, because the reported absorbance value is too. Beer Lambert Law Ap Chemistry Questions.
From www.chegg.com
Solved SUR o X CHEM 242 Online Lab 3 BeerLambert Law Beer Lambert Law Ap Chemistry Questions What will be the units of ∈, the specific. (a) the longest wavelength of. Study with quizlet and memorize flashcards containing terms like beers law equation, color wavelengths, absorbance and more. [1] the absorbance is a dimensionless quantity, that is, a quantity without units. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is.. Beer Lambert Law Ap Chemistry Questions.
From www.studocu.com
BeerLambert Law Lecture notes with annotations CHEM0020 Studocu Beer Lambert Law Ap Chemistry Questions (a) the longest wavelength of. Study with quizlet and memorize flashcards containing terms like beers law equation, color wavelengths, absorbance and more. The actual solution concentration must have been lower than 0.150m, because the reported absorbance value is too small based on the. Answer the following questions regarding light and its interactions with molecules, atoms, and ions. In most experiments,. Beer Lambert Law Ap Chemistry Questions.
From study.com
How to Find the Absorbance of a Solution Using the BeerLambert Law Beer Lambert Law Ap Chemistry Questions In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Answer the following questions regarding light and its interactions with molecules, atoms, and ions. [1] the absorbance is a dimensionless quantity, that is, a quantity without units. What will be the units of ∈, the specific. (a) the longest wavelength of. Study with quizlet. Beer Lambert Law Ap Chemistry Questions.
From hudsontinhoffman.blogspot.com
Beer's Lambert Law Equation HudsontinHoffman Beer Lambert Law Ap Chemistry Questions The actual solution concentration must have been lower than 0.150m, because the reported absorbance value is too small based on the. [1] the absorbance is a dimensionless quantity, that is, a quantity without units. (a) the longest wavelength of. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. What will be the units. Beer Lambert Law Ap Chemistry Questions.
From rumble.com
BeerLambert Law, Spectrophotometry, Example Chemistry Beer Lambert Law Ap Chemistry Questions Answer the following questions regarding light and its interactions with molecules, atoms, and ions. [1] the absorbance is a dimensionless quantity, that is, a quantity without units. Study with quizlet and memorize flashcards containing terms like beers law equation, color wavelengths, absorbance and more. (a) the longest wavelength of. What will be the units of ∈, the specific. In most. Beer Lambert Law Ap Chemistry Questions.
From www.youtube.com
Spectrophotometry and the BeerLambert Law AP Chem Unit 3, Topic 13 Beer Lambert Law Ap Chemistry Questions Study with quizlet and memorize flashcards containing terms like beers law equation, color wavelengths, absorbance and more. (a) the longest wavelength of. [1] the absorbance is a dimensionless quantity, that is, a quantity without units. The actual solution concentration must have been lower than 0.150m, because the reported absorbance value is too small based on the. In most experiments, molar. Beer Lambert Law Ap Chemistry Questions.
From www.iitianacademy.com
AP Chemistry 3.13 BeerLambert Law Exam Style questions with Answer MCQ Beer Lambert Law Ap Chemistry Questions What will be the units of ∈, the specific. The actual solution concentration must have been lower than 0.150m, because the reported absorbance value is too small based on the. Answer the following questions regarding light and its interactions with molecules, atoms, and ions. Study with quizlet and memorize flashcards containing terms like beers law equation, color wavelengths, absorbance and. Beer Lambert Law Ap Chemistry Questions.
From www.youtube.com
3.13. BeerLambert Law College Board AP Chemistry YouTube Beer Lambert Law Ap Chemistry Questions The actual solution concentration must have been lower than 0.150m, because the reported absorbance value is too small based on the. Study with quizlet and memorize flashcards containing terms like beers law equation, color wavelengths, absorbance and more. Answer the following questions regarding light and its interactions with molecules, atoms, and ions. [1] the absorbance is a dimensionless quantity, that. Beer Lambert Law Ap Chemistry Questions.
From www.chegg.com
Solved The BeerLambert Law is useful for determining the Beer Lambert Law Ap Chemistry Questions [1] the absorbance is a dimensionless quantity, that is, a quantity without units. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Answer the following questions regarding light and its interactions with molecules, atoms, and ions. (a) the longest wavelength of. Study with quizlet and memorize flashcards containing terms like beers law equation,. Beer Lambert Law Ap Chemistry Questions.
From www.youtube.com
Beer Lambert's Law, Absorbance & Transmittance Spectrophotometry Beer Lambert Law Ap Chemistry Questions (a) the longest wavelength of. Study with quizlet and memorize flashcards containing terms like beers law equation, color wavelengths, absorbance and more. The actual solution concentration must have been lower than 0.150m, because the reported absorbance value is too small based on the. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Answer. Beer Lambert Law Ap Chemistry Questions.
From www.thoughtco.com
Beer's Law Definition and Equation Beer Lambert Law Ap Chemistry Questions [1] the absorbance is a dimensionless quantity, that is, a quantity without units. Answer the following questions regarding light and its interactions with molecules, atoms, and ions. (a) the longest wavelength of. Study with quizlet and memorize flashcards containing terms like beers law equation, color wavelengths, absorbance and more. In most experiments, molar absorptivity (ε) and the length (b) are. Beer Lambert Law Ap Chemistry Questions.