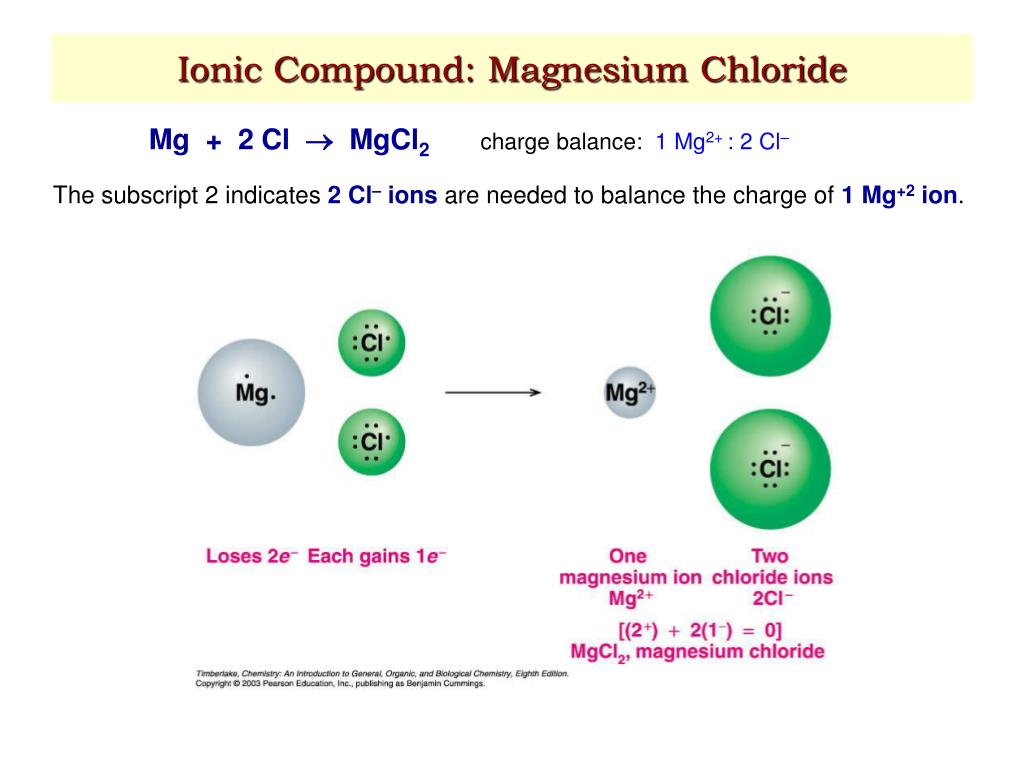
Magnesium Chloride Ionic Bond . Magnesium and nitrogen react to form an ionic compound. Mg forms ionic bond to cl by donating its valence electrons to two cl atoms. Again, noble gas structures are formed, and the magnesium oxide is held together by very strong attractions. The electron configuration of mg is [ne]3s². Compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic bonds: At r0, the ions are more stable (have a lower potential energy) than they are at an infinite internuclear distance. We summarize the important points about ionic bonding: Predict which forms an anion, which forms a cation, and the charges of each ion. Write the symbol for each ion and name them.
from www.slideserve.com
The electron configuration of mg is [ne]3s². We summarize the important points about ionic bonding: Predict which forms an anion, which forms a cation, and the charges of each ion. At r0, the ions are more stable (have a lower potential energy) than they are at an infinite internuclear distance. Mg forms ionic bond to cl by donating its valence electrons to two cl atoms. Compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic bonds: Again, noble gas structures are formed, and the magnesium oxide is held together by very strong attractions. Write the symbol for each ion and name them. Magnesium and nitrogen react to form an ionic compound.
PPT Chapter 4 Compounds and Their Bonds PowerPoint Presentation
Magnesium Chloride Ionic Bond We summarize the important points about ionic bonding: We summarize the important points about ionic bonding: Mg forms ionic bond to cl by donating its valence electrons to two cl atoms. At r0, the ions are more stable (have a lower potential energy) than they are at an infinite internuclear distance. Magnesium and nitrogen react to form an ionic compound. Compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic bonds: Predict which forms an anion, which forms a cation, and the charges of each ion. Write the symbol for each ion and name them. The electron configuration of mg is [ne]3s². Again, noble gas structures are formed, and the magnesium oxide is held together by very strong attractions.
From blog.iceslicer.com
Chloride Spotlight What is Magnesium Chloride? Magnesium Chloride Ionic Bond At r0, the ions are more stable (have a lower potential energy) than they are at an infinite internuclear distance. Predict which forms an anion, which forms a cation, and the charges of each ion. Again, noble gas structures are formed, and the magnesium oxide is held together by very strong attractions. Magnesium and nitrogen react to form an ionic. Magnesium Chloride Ionic Bond.
From schematicpartebriose.z14.web.core.windows.net
Diagram Of Ionic Bonding In Sodium Chloride Magnesium Chloride Ionic Bond Magnesium and nitrogen react to form an ionic compound. The electron configuration of mg is [ne]3s². Predict which forms an anion, which forms a cation, and the charges of each ion. We summarize the important points about ionic bonding: Mg forms ionic bond to cl by donating its valence electrons to two cl atoms. Write the symbol for each ion. Magnesium Chloride Ionic Bond.
From www.slideshare.net
2012 topic 4.1 bonding ionic Magnesium Chloride Ionic Bond At r0, the ions are more stable (have a lower potential energy) than they are at an infinite internuclear distance. Compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic bonds: Magnesium and nitrogen react to form an ionic compound. Write the symbol for each ion and name them. Again, noble. Magnesium Chloride Ionic Bond.
From www.youtube.com
Is MgCl2 (Magnesium chloride) Ionic or Covalent? YouTube Magnesium Chloride Ionic Bond The electron configuration of mg is [ne]3s². Compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic bonds: We summarize the important points about ionic bonding: At r0, the ions are more stable (have a lower potential energy) than they are at an infinite internuclear distance. Again, noble gas structures are. Magnesium Chloride Ionic Bond.
From www.slideserve.com
PPT Chapter 6 Ionic Compounds PowerPoint Presentation, free Magnesium Chloride Ionic Bond Mg forms ionic bond to cl by donating its valence electrons to two cl atoms. Predict which forms an anion, which forms a cation, and the charges of each ion. Write the symbol for each ion and name them. Again, noble gas structures are formed, and the magnesium oxide is held together by very strong attractions. We summarize the important. Magnesium Chloride Ionic Bond.
From www.youtube.com
IONIC BOND FORMATION OF MAGNESIUM CHLORIDE AND SODIUM CHLORIDE YouTube Magnesium Chloride Ionic Bond Compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic bonds: The electron configuration of mg is [ne]3s². Predict which forms an anion, which forms a cation, and the charges of each ion. At r0, the ions are more stable (have a lower potential energy) than they are at an infinite. Magnesium Chloride Ionic Bond.
From www.chemicalslearning.com
Magnesium Chloride Formula, Properties and Uses Magnesium Chloride Ionic Bond At r0, the ions are more stable (have a lower potential energy) than they are at an infinite internuclear distance. Magnesium and nitrogen react to form an ionic compound. Compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic bonds: The electron configuration of mg is [ne]3s². Predict which forms an. Magnesium Chloride Ionic Bond.
From www.edplace.com
Explain Ionic bonding Worksheet EdPlace Magnesium Chloride Ionic Bond Compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic bonds: At r0, the ions are more stable (have a lower potential energy) than they are at an infinite internuclear distance. Again, noble gas structures are formed, and the magnesium oxide is held together by very strong attractions. We summarize the. Magnesium Chloride Ionic Bond.
From www.elektramagnesium.com.au
Ionic Magnesium Chloride Hexahydrate or Chelated Magnesium? Elektra Magnesium Chloride Ionic Bond At r0, the ions are more stable (have a lower potential energy) than they are at an infinite internuclear distance. We summarize the important points about ionic bonding: Compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic bonds: Again, noble gas structures are formed, and the magnesium oxide is held. Magnesium Chloride Ionic Bond.
From diagramedia.blogspot.com
Electron Dot Diagram For Magnesium Chloride Diagram Media Magnesium Chloride Ionic Bond Predict which forms an anion, which forms a cation, and the charges of each ion. Magnesium and nitrogen react to form an ionic compound. Again, noble gas structures are formed, and the magnesium oxide is held together by very strong attractions. Compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic. Magnesium Chloride Ionic Bond.
From www.slideserve.com
PPT Visualizing a Chemical Reaction PowerPoint Presentation, free Magnesium Chloride Ionic Bond We summarize the important points about ionic bonding: At r0, the ions are more stable (have a lower potential energy) than they are at an infinite internuclear distance. Predict which forms an anion, which forms a cation, and the charges of each ion. The electron configuration of mg is [ne]3s². Write the symbol for each ion and name them. Mg. Magnesium Chloride Ionic Bond.
From www.slideserve.com
PPT Ionic Bonds and Ionic Compounds PowerPoint Presentation, free Magnesium Chloride Ionic Bond Write the symbol for each ion and name them. Predict which forms an anion, which forms a cation, and the charges of each ion. The electron configuration of mg is [ne]3s². Compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic bonds: Magnesium and nitrogen react to form an ionic compound.. Magnesium Chloride Ionic Bond.
From ar.inspiredpencil.com
Ionic Bonding Mgcl2 Magnesium Chloride Ionic Bond We summarize the important points about ionic bonding: Predict which forms an anion, which forms a cation, and the charges of each ion. Mg forms ionic bond to cl by donating its valence electrons to two cl atoms. Magnesium and nitrogen react to form an ionic compound. At r0, the ions are more stable (have a lower potential energy) than. Magnesium Chloride Ionic Bond.
From schematicmegaul3k.z21.web.core.windows.net
Dot And Cross Diagram For Magnesium Chloride Magnesium Chloride Ionic Bond Magnesium and nitrogen react to form an ionic compound. The electron configuration of mg is [ne]3s². At r0, the ions are more stable (have a lower potential energy) than they are at an infinite internuclear distance. Predict which forms an anion, which forms a cation, and the charges of each ion. We summarize the important points about ionic bonding: Again,. Magnesium Chloride Ionic Bond.
From brainly.in
What is ionic bond order formation of magnesium chloride? Brainly.in Magnesium Chloride Ionic Bond Predict which forms an anion, which forms a cation, and the charges of each ion. Mg forms ionic bond to cl by donating its valence electrons to two cl atoms. We summarize the important points about ionic bonding: Magnesium and nitrogen react to form an ionic compound. Write the symbol for each ion and name them. Compounds composed of ions. Magnesium Chloride Ionic Bond.
From www.knowledgeboat.com
Explain with the help of (i) an ionic equation (ii) KnowledgeBoat Magnesium Chloride Ionic Bond Again, noble gas structures are formed, and the magnesium oxide is held together by very strong attractions. Write the symbol for each ion and name them. At r0, the ions are more stable (have a lower potential energy) than they are at an infinite internuclear distance. Predict which forms an anion, which forms a cation, and the charges of each. Magnesium Chloride Ionic Bond.
From www.slideshare.net
Chem matters ch6_ionic_bond Magnesium Chloride Ionic Bond Magnesium and nitrogen react to form an ionic compound. We summarize the important points about ionic bonding: Compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic bonds: Predict which forms an anion, which forms a cation, and the charges of each ion. The electron configuration of mg is [ne]3s². Mg. Magnesium Chloride Ionic Bond.
From www.science-revision.co.uk
Introduction to ionic bonding Magnesium Chloride Ionic Bond Write the symbol for each ion and name them. At r0, the ions are more stable (have a lower potential energy) than they are at an infinite internuclear distance. The electron configuration of mg is [ne]3s². Magnesium and nitrogen react to form an ionic compound. Again, noble gas structures are formed, and the magnesium oxide is held together by very. Magnesium Chloride Ionic Bond.
From guidedehartchoultries.z21.web.core.windows.net
Lewis Dot Diagram For Magnesium Chloride Magnesium Chloride Ionic Bond The electron configuration of mg is [ne]3s². Compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic bonds: Mg forms ionic bond to cl by donating its valence electrons to two cl atoms. At r0, the ions are more stable (have a lower potential energy) than they are at an infinite. Magnesium Chloride Ionic Bond.
From www.slideserve.com
PPT Chapter 4 Compounds and Their Bonds PowerPoint Presentation Magnesium Chloride Ionic Bond Write the symbol for each ion and name them. Magnesium and nitrogen react to form an ionic compound. We summarize the important points about ionic bonding: Mg forms ionic bond to cl by donating its valence electrons to two cl atoms. The electron configuration of mg is [ne]3s². At r0, the ions are more stable (have a lower potential energy). Magnesium Chloride Ionic Bond.
From brainly.in
Explain the formation of MgCl2 with the help of electron dot structure Magnesium Chloride Ionic Bond Again, noble gas structures are formed, and the magnesium oxide is held together by very strong attractions. Write the symbol for each ion and name them. At r0, the ions are more stable (have a lower potential energy) than they are at an infinite internuclear distance. Mg forms ionic bond to cl by donating its valence electrons to two cl. Magnesium Chloride Ionic Bond.
From techiescientist.com
Is MgCl2 Ionic or Covalent? Techiescientist Magnesium Chloride Ionic Bond Again, noble gas structures are formed, and the magnesium oxide is held together by very strong attractions. Predict which forms an anion, which forms a cation, and the charges of each ion. Mg forms ionic bond to cl by donating its valence electrons to two cl atoms. At r0, the ions are more stable (have a lower potential energy) than. Magnesium Chloride Ionic Bond.
From enginedatanichered.z21.web.core.windows.net
Atomic Diagram Of Magnesium Magnesium Chloride Ionic Bond Again, noble gas structures are formed, and the magnesium oxide is held together by very strong attractions. Predict which forms an anion, which forms a cation, and the charges of each ion. At r0, the ions are more stable (have a lower potential energy) than they are at an infinite internuclear distance. Mg forms ionic bond to cl by donating. Magnesium Chloride Ionic Bond.
From www.youtube.com
Ionic bonding Sodium chloride,Magnesium chloride Class 10 Magnesium Chloride Ionic Bond Predict which forms an anion, which forms a cation, and the charges of each ion. At r0, the ions are more stable (have a lower potential energy) than they are at an infinite internuclear distance. Compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic bonds: Write the symbol for each. Magnesium Chloride Ionic Bond.
From www.elevise.co.uk
C2 A) Ionic Bonds AQA Combined Science Trilogy Elevise Magnesium Chloride Ionic Bond Again, noble gas structures are formed, and the magnesium oxide is held together by very strong attractions. At r0, the ions are more stable (have a lower potential energy) than they are at an infinite internuclear distance. Magnesium and nitrogen react to form an ionic compound. The electron configuration of mg is [ne]3s². Compounds composed of ions are called ionic. Magnesium Chloride Ionic Bond.
From montessorimuddle.org
An Introduction to Ionic Bonding Montessori Muddle Magnesium Chloride Ionic Bond The electron configuration of mg is [ne]3s². Compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic bonds: Mg forms ionic bond to cl by donating its valence electrons to two cl atoms. Magnesium and nitrogen react to form an ionic compound. Again, noble gas structures are formed, and the magnesium. Magnesium Chloride Ionic Bond.
From manualdatadewsbury.z13.web.core.windows.net
Dot Diagram For Mg Magnesium Chloride Ionic Bond The electron configuration of mg is [ne]3s². We summarize the important points about ionic bonding: Magnesium and nitrogen react to form an ionic compound. Again, noble gas structures are formed, and the magnesium oxide is held together by very strong attractions. Write the symbol for each ion and name them. Predict which forms an anion, which forms a cation, and. Magnesium Chloride Ionic Bond.
From www.freeexamacademy.com
Atoms, Elements And Compounds Free Exam Academy Magnesium Chloride Ionic Bond Again, noble gas structures are formed, and the magnesium oxide is held together by very strong attractions. Predict which forms an anion, which forms a cation, and the charges of each ion. The electron configuration of mg is [ne]3s². We summarize the important points about ionic bonding: Magnesium and nitrogen react to form an ionic compound. Compounds composed of ions. Magnesium Chloride Ionic Bond.
From www.youtube.com
Draw the Lewis Structure of MgCl2 (magnesium chloride) YouTube Magnesium Chloride Ionic Bond Compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic bonds: The electron configuration of mg is [ne]3s². Write the symbol for each ion and name them. Predict which forms an anion, which forms a cation, and the charges of each ion. Again, noble gas structures are formed, and the magnesium. Magnesium Chloride Ionic Bond.
From slidetodoc.com
Ionic Bonding Elements are the simplest substances There Magnesium Chloride Ionic Bond Magnesium and nitrogen react to form an ionic compound. The electron configuration of mg is [ne]3s². Again, noble gas structures are formed, and the magnesium oxide is held together by very strong attractions. Write the symbol for each ion and name them. Compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by. Magnesium Chloride Ionic Bond.
From www.elevise.co.uk
C2 A) Ionic Bonds AQA Combined Science Trilogy Elevise Magnesium Chloride Ionic Bond At r0, the ions are more stable (have a lower potential energy) than they are at an infinite internuclear distance. The electron configuration of mg is [ne]3s². Magnesium and nitrogen react to form an ionic compound. Predict which forms an anion, which forms a cation, and the charges of each ion. Write the symbol for each ion and name them.. Magnesium Chloride Ionic Bond.
From www.youtube.com
How to draw dot and cross diagram of magnesium chloride ionic compound Magnesium Chloride Ionic Bond The electron configuration of mg is [ne]3s². Predict which forms an anion, which forms a cation, and the charges of each ion. Magnesium and nitrogen react to form an ionic compound. At r0, the ions are more stable (have a lower potential energy) than they are at an infinite internuclear distance. Again, noble gas structures are formed, and the magnesium. Magnesium Chloride Ionic Bond.
From www.slideshare.net
2012 topic 4.1 bonding ionic Magnesium Chloride Ionic Bond Mg forms ionic bond to cl by donating its valence electrons to two cl atoms. Again, noble gas structures are formed, and the magnesium oxide is held together by very strong attractions. The electron configuration of mg is [ne]3s². At r0, the ions are more stable (have a lower potential energy) than they are at an infinite internuclear distance. Predict. Magnesium Chloride Ionic Bond.
From byjus.com
By the transfer of electrons, illustrate the formation of bond in Magnesium Chloride Ionic Bond Mg forms ionic bond to cl by donating its valence electrons to two cl atoms. Again, noble gas structures are formed, and the magnesium oxide is held together by very strong attractions. The electron configuration of mg is [ne]3s². Magnesium and nitrogen react to form an ionic compound. We summarize the important points about ionic bonding: Write the symbol for. Magnesium Chloride Ionic Bond.
From www.slideserve.com
PPT Ionic Bonding PowerPoint Presentation, free download ID2435173 Magnesium Chloride Ionic Bond Magnesium and nitrogen react to form an ionic compound. Predict which forms an anion, which forms a cation, and the charges of each ion. At r0, the ions are more stable (have a lower potential energy) than they are at an infinite internuclear distance. Mg forms ionic bond to cl by donating its valence electrons to two cl atoms. Again,. Magnesium Chloride Ionic Bond.