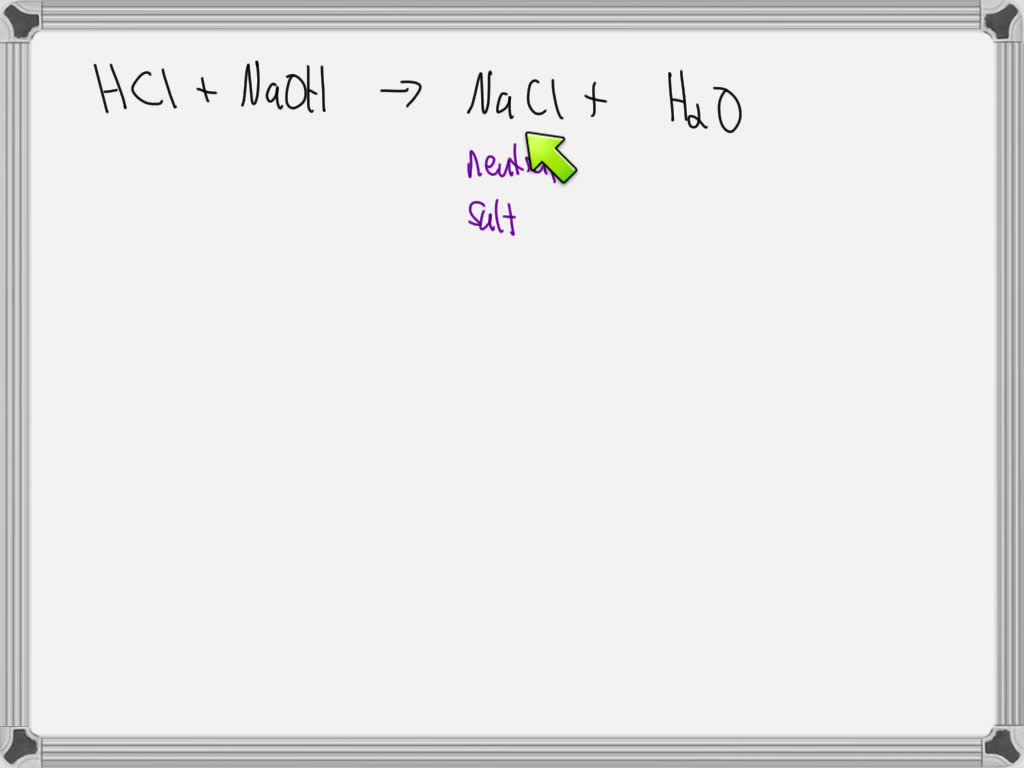Titration Hcl Naoh Berechnung . Titrationskurve starke säure mit starker base. Naoh + hcl = nacl + h 2 o. Hcl and naoh are strong acid and strong base respectively and their titration curves are similar (shape of curve) in different concentrations. Berechne eine unbekannte konzentration von 0,15 ml hcl: Die titrationskurve einer starken säure mit einer starken base ergibt sich bei der titration von. Determination of sodium hydroxide concentration is about as. Let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a strong base (1.0 m naoh). Titration of sodium hydroxide with hydrochloric acid. Using the formula c1v1 = c2v2, where c1 is the molarity of the naoh, the difference in. With this data, the volume of naoh used can be calculated.
from www.numerade.com
Using the formula c1v1 = c2v2, where c1 is the molarity of the naoh, the difference in. With this data, the volume of naoh used can be calculated. Naoh + hcl = nacl + h 2 o. Let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a strong base (1.0 m naoh). Hcl and naoh are strong acid and strong base respectively and their titration curves are similar (shape of curve) in different concentrations. Titration of sodium hydroxide with hydrochloric acid. Die titrationskurve einer starken säure mit einer starken base ergibt sich bei der titration von. Berechne eine unbekannte konzentration von 0,15 ml hcl: Determination of sodium hydroxide concentration is about as. Titrationskurve starke säure mit starker base.
SOLVED HCl + NaOH > H2O + NaCl. What is the pH of the solution at the
Titration Hcl Naoh Berechnung Naoh + hcl = nacl + h 2 o. Berechne eine unbekannte konzentration von 0,15 ml hcl: Using the formula c1v1 = c2v2, where c1 is the molarity of the naoh, the difference in. Let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a strong base (1.0 m naoh). Titration of sodium hydroxide with hydrochloric acid. Determination of sodium hydroxide concentration is about as. With this data, the volume of naoh used can be calculated. Titrationskurve starke säure mit starker base. Die titrationskurve einer starken säure mit einer starken base ergibt sich bei der titration von. Naoh + hcl = nacl + h 2 o. Hcl and naoh are strong acid and strong base respectively and their titration curves are similar (shape of curve) in different concentrations.
From www.numerade.com
SOLVED Consider the model of titrating hydrochloric acid in the given Titration Hcl Naoh Berechnung With this data, the volume of naoh used can be calculated. Titration of sodium hydroxide with hydrochloric acid. Determination of sodium hydroxide concentration is about as. Naoh + hcl = nacl + h 2 o. Let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a strong base (1.0 m naoh). Berechne eine unbekannte konzentration von. Titration Hcl Naoh Berechnung.
From www.reddit.com
HCl + NaOH titration calculation correct? r/chemhelp Titration Hcl Naoh Berechnung Let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a strong base (1.0 m naoh). Titrationskurve starke säure mit starker base. Using the formula c1v1 = c2v2, where c1 is the molarity of the naoh, the difference in. Die titrationskurve einer starken säure mit einer starken base ergibt sich bei der titration von. Determination of. Titration Hcl Naoh Berechnung.
From mungfali.com
HCl NaOH Titration Titration Hcl Naoh Berechnung With this data, the volume of naoh used can be calculated. Die titrationskurve einer starken säure mit einer starken base ergibt sich bei der titration von. Let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a strong base (1.0 m naoh). Determination of sodium hydroxide concentration is about as. Hcl and naoh are strong acid. Titration Hcl Naoh Berechnung.
From www.learnsci.com
LearnSci Smart Worksheet Determine Concentration of HCl by Titration Titration Hcl Naoh Berechnung Die titrationskurve einer starken säure mit einer starken base ergibt sich bei der titration von. Hcl and naoh are strong acid and strong base respectively and their titration curves are similar (shape of curve) in different concentrations. Determination of sodium hydroxide concentration is about as. Titration of sodium hydroxide with hydrochloric acid. With this data, the volume of naoh used. Titration Hcl Naoh Berechnung.
From studylib.net
Titration of HCl with NaOH Titration Hcl Naoh Berechnung Titration of sodium hydroxide with hydrochloric acid. Using the formula c1v1 = c2v2, where c1 is the molarity of the naoh, the difference in. Naoh + hcl = nacl + h 2 o. Die titrationskurve einer starken säure mit einer starken base ergibt sich bei der titration von. Berechne eine unbekannte konzentration von 0,15 ml hcl: With this data, the. Titration Hcl Naoh Berechnung.
From www.youtube.com
Conductometric titration I strong acid (HCl) versus strong base Titration Hcl Naoh Berechnung Berechne eine unbekannte konzentration von 0,15 ml hcl: Hcl and naoh are strong acid and strong base respectively and their titration curves are similar (shape of curve) in different concentrations. Naoh + hcl = nacl + h 2 o. Die titrationskurve einer starken säure mit einer starken base ergibt sich bei der titration von. With this data, the volume of. Titration Hcl Naoh Berechnung.
From dokumen.tips
(PDF) AcidBase Titration NaOH with HCL DOKUMEN.TIPS Titration Hcl Naoh Berechnung Hcl and naoh are strong acid and strong base respectively and their titration curves are similar (shape of curve) in different concentrations. Berechne eine unbekannte konzentration von 0,15 ml hcl: With this data, the volume of naoh used can be calculated. Die titrationskurve einer starken säure mit einer starken base ergibt sich bei der titration von. Titrationskurve starke säure mit. Titration Hcl Naoh Berechnung.
From www.chegg.com
Solved Method Titration of sodium hydroxide and hydrochloric Titration Hcl Naoh Berechnung Hcl and naoh are strong acid and strong base respectively and their titration curves are similar (shape of curve) in different concentrations. Berechne eine unbekannte konzentration von 0,15 ml hcl: Titrationskurve starke säure mit starker base. Titration of sodium hydroxide with hydrochloric acid. Determination of sodium hydroxide concentration is about as. Using the formula c1v1 = c2v2, where c1 is. Titration Hcl Naoh Berechnung.
From www.numerade.com
SOLVED The following data were collected during a titration Calculate Titration Hcl Naoh Berechnung Titration of sodium hydroxide with hydrochloric acid. Determination of sodium hydroxide concentration is about as. Using the formula c1v1 = c2v2, where c1 is the molarity of the naoh, the difference in. Let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a strong base (1.0 m naoh). Naoh + hcl = nacl + h 2. Titration Hcl Naoh Berechnung.
From www.youtube.com
Titration HCl and NaOH methyl orange YouTube Titration Hcl Naoh Berechnung With this data, the volume of naoh used can be calculated. Using the formula c1v1 = c2v2, where c1 is the molarity of the naoh, the difference in. Titrationskurve starke säure mit starker base. Determination of sodium hydroxide concentration is about as. Let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a strong base (1.0. Titration Hcl Naoh Berechnung.
From www.numerade.com
SOLVED When examining the titrations of hydrochloric acid and acetic Titration Hcl Naoh Berechnung Using the formula c1v1 = c2v2, where c1 is the molarity of the naoh, the difference in. Berechne eine unbekannte konzentration von 0,15 ml hcl: Die titrationskurve einer starken säure mit einer starken base ergibt sich bei der titration von. Let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a strong base (1.0 m naoh).. Titration Hcl Naoh Berechnung.
From mungfali.com
NaOH HCl Titration Curve Titration Hcl Naoh Berechnung Naoh + hcl = nacl + h 2 o. Let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a strong base (1.0 m naoh). Die titrationskurve einer starken säure mit einer starken base ergibt sich bei der titration von. Titration of sodium hydroxide with hydrochloric acid. Titrationskurve starke säure mit starker base. Berechne eine unbekannte. Titration Hcl Naoh Berechnung.
From www.numerade.com
SOLVED 4) (4 pts) In lab you titrated HCl with NaOH Sketch the Titration Hcl Naoh Berechnung Determination of sodium hydroxide concentration is about as. With this data, the volume of naoh used can be calculated. Titration of sodium hydroxide with hydrochloric acid. Using the formula c1v1 = c2v2, where c1 is the molarity of the naoh, the difference in. Hcl and naoh are strong acid and strong base respectively and their titration curves are similar (shape. Titration Hcl Naoh Berechnung.
From mungfali.com
HCl NaOH Titration Titration Hcl Naoh Berechnung With this data, the volume of naoh used can be calculated. Titration of sodium hydroxide with hydrochloric acid. Berechne eine unbekannte konzentration von 0,15 ml hcl: Titrationskurve starke säure mit starker base. Using the formula c1v1 = c2v2, where c1 is the molarity of the naoh, the difference in. Determination of sodium hydroxide concentration is about as. Let's assume you. Titration Hcl Naoh Berechnung.
From mungfali.com
Titration Curve HCl And NaOH Titration Hcl Naoh Berechnung Hcl and naoh are strong acid and strong base respectively and their titration curves are similar (shape of curve) in different concentrations. Titration of sodium hydroxide with hydrochloric acid. Naoh + hcl = nacl + h 2 o. With this data, the volume of naoh used can be calculated. Using the formula c1v1 = c2v2, where c1 is the molarity. Titration Hcl Naoh Berechnung.
From mungfali.com
HCl NaOH Titration Titration Hcl Naoh Berechnung Die titrationskurve einer starken säure mit einer starken base ergibt sich bei der titration von. Using the formula c1v1 = c2v2, where c1 is the molarity of the naoh, the difference in. Titration of sodium hydroxide with hydrochloric acid. Berechne eine unbekannte konzentration von 0,15 ml hcl: Naoh + hcl = nacl + h 2 o. Titrationskurve starke säure mit. Titration Hcl Naoh Berechnung.
From www.numerade.com
Lab Titration of HCl and NaOH to Determine the Concentration of NaOH Titration Hcl Naoh Berechnung Titration of sodium hydroxide with hydrochloric acid. Titrationskurve starke säure mit starker base. Determination of sodium hydroxide concentration is about as. Hcl and naoh are strong acid and strong base respectively and their titration curves are similar (shape of curve) in different concentrations. With this data, the volume of naoh used can be calculated. Using the formula c1v1 = c2v2,. Titration Hcl Naoh Berechnung.
From studylib.net
3. Titration NaOH and HCl concentration of an alkali Titration Hcl Naoh Berechnung Let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a strong base (1.0 m naoh). Naoh + hcl = nacl + h 2 o. Hcl and naoh are strong acid and strong base respectively and their titration curves are similar (shape of curve) in different concentrations. Berechne eine unbekannte konzentration von 0,15 ml hcl: Using. Titration Hcl Naoh Berechnung.
From psiberg.com
The Equivalence Point Acid/Base Titrations PSIBERG Titration Hcl Naoh Berechnung Titration of sodium hydroxide with hydrochloric acid. Let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a strong base (1.0 m naoh). Berechne eine unbekannte konzentration von 0,15 ml hcl: Hcl and naoh are strong acid and strong base respectively and their titration curves are similar (shape of curve) in different concentrations. Naoh + hcl. Titration Hcl Naoh Berechnung.
From www.numerade.com
SOLVED Determine the molarity of a 23.00 ml HCl solution if it takes Titration Hcl Naoh Berechnung Titrationskurve starke säure mit starker base. Berechne eine unbekannte konzentration von 0,15 ml hcl: Let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a strong base (1.0 m naoh). Titration of sodium hydroxide with hydrochloric acid. Using the formula c1v1 = c2v2, where c1 is the molarity of the naoh, the difference in. Die titrationskurve. Titration Hcl Naoh Berechnung.
From jeanius123.weebly.com
Chemistry Titration of HCl using NaOH Titration Hcl Naoh Berechnung With this data, the volume of naoh used can be calculated. Titration of sodium hydroxide with hydrochloric acid. Let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a strong base (1.0 m naoh). Using the formula c1v1 = c2v2, where c1 is the molarity of the naoh, the difference in. Die titrationskurve einer starken säure. Titration Hcl Naoh Berechnung.
From mungfali.com
HCl NaOH Titration Titration Hcl Naoh Berechnung With this data, the volume of naoh used can be calculated. Hcl and naoh are strong acid and strong base respectively and their titration curves are similar (shape of curve) in different concentrations. Die titrationskurve einer starken säure mit einer starken base ergibt sich bei der titration von. Using the formula c1v1 = c2v2, where c1 is the molarity of. Titration Hcl Naoh Berechnung.
From mungfali.com
HCl NaOH Titration Titration Hcl Naoh Berechnung Naoh + hcl = nacl + h 2 o. Titrationskurve starke säure mit starker base. Determination of sodium hydroxide concentration is about as. Berechne eine unbekannte konzentration von 0,15 ml hcl: Hcl and naoh are strong acid and strong base respectively and their titration curves are similar (shape of curve) in different concentrations. Die titrationskurve einer starken säure mit einer. Titration Hcl Naoh Berechnung.
From studylib.net
Titration of Hydrochloric Acid with Sodium Hydroxide Titration Hcl Naoh Berechnung Let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a strong base (1.0 m naoh). Hcl and naoh are strong acid and strong base respectively and their titration curves are similar (shape of curve) in different concentrations. Determination of sodium hydroxide concentration is about as. Naoh + hcl = nacl + h 2 o. Titration. Titration Hcl Naoh Berechnung.
From byjus.com
The graph of pH during the titration of NaOH and HCl Titration Hcl Naoh Berechnung Let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a strong base (1.0 m naoh). Using the formula c1v1 = c2v2, where c1 is the molarity of the naoh, the difference in. Determination of sodium hydroxide concentration is about as. Berechne eine unbekannte konzentration von 0,15 ml hcl: Titrationskurve starke säure mit starker base. Naoh. Titration Hcl Naoh Berechnung.
From www.studyhelp.de
Titration StudyHelp OnlineLernen Titration Hcl Naoh Berechnung Determination of sodium hydroxide concentration is about as. Naoh + hcl = nacl + h 2 o. Using the formula c1v1 = c2v2, where c1 is the molarity of the naoh, the difference in. Let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a strong base (1.0 m naoh). Titration of sodium hydroxide with hydrochloric. Titration Hcl Naoh Berechnung.
From www.researchgate.net
Titration of HCl (0.1M) against NaOH (0.1M) Download Scientific Diagram Titration Hcl Naoh Berechnung Naoh + hcl = nacl + h 2 o. Using the formula c1v1 = c2v2, where c1 is the molarity of the naoh, the difference in. Die titrationskurve einer starken säure mit einer starken base ergibt sich bei der titration von. Titration of sodium hydroxide with hydrochloric acid. Hcl and naoh are strong acid and strong base respectively and their. Titration Hcl Naoh Berechnung.
From www.numerade.com
SOLVED HCl + NaOH > H2O + NaCl. What is the pH of the solution at the Titration Hcl Naoh Berechnung Determination of sodium hydroxide concentration is about as. Titrationskurve starke säure mit starker base. Naoh + hcl = nacl + h 2 o. With this data, the volume of naoh used can be calculated. Using the formula c1v1 = c2v2, where c1 is the molarity of the naoh, the difference in. Die titrationskurve einer starken säure mit einer starken base. Titration Hcl Naoh Berechnung.
From www.visionlearning.com
Acids and Bases I Math in Science Visionlearning Titration Hcl Naoh Berechnung Hcl and naoh are strong acid and strong base respectively and their titration curves are similar (shape of curve) in different concentrations. Titration of sodium hydroxide with hydrochloric acid. Berechne eine unbekannte konzentration von 0,15 ml hcl: Die titrationskurve einer starken säure mit einer starken base ergibt sich bei der titration von. Using the formula c1v1 = c2v2, where c1. Titration Hcl Naoh Berechnung.
From mungfali.com
HCl NaOH Titration Titration Hcl Naoh Berechnung Titration of sodium hydroxide with hydrochloric acid. Hcl and naoh are strong acid and strong base respectively and their titration curves are similar (shape of curve) in different concentrations. Determination of sodium hydroxide concentration is about as. Titrationskurve starke säure mit starker base. With this data, the volume of naoh used can be calculated. Let's assume you are titrating a. Titration Hcl Naoh Berechnung.
From mungfali.com
HCl NaOH Titration Titration Hcl Naoh Berechnung Hcl and naoh are strong acid and strong base respectively and their titration curves are similar (shape of curve) in different concentrations. Titrationskurve starke säure mit starker base. Determination of sodium hydroxide concentration is about as. Using the formula c1v1 = c2v2, where c1 is the molarity of the naoh, the difference in. Titration of sodium hydroxide with hydrochloric acid.. Titration Hcl Naoh Berechnung.
From www.youtube.com
Titration calculation 1 NaOH Vs HCl YouTube Titration Hcl Naoh Berechnung Titration of sodium hydroxide with hydrochloric acid. With this data, the volume of naoh used can be calculated. Hcl and naoh are strong acid and strong base respectively and their titration curves are similar (shape of curve) in different concentrations. Determination of sodium hydroxide concentration is about as. Titrationskurve starke säure mit starker base. Berechne eine unbekannte konzentration von 0,15. Titration Hcl Naoh Berechnung.
From www.chegg.com
Solved Titration of HCl with standardized NaOH Titration Hcl Naoh Berechnung Let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a strong base (1.0 m naoh). Using the formula c1v1 = c2v2, where c1 is the molarity of the naoh, the difference in. Die titrationskurve einer starken säure mit einer starken base ergibt sich bei der titration von. Titrationskurve starke säure mit starker base. Naoh +. Titration Hcl Naoh Berechnung.
From www.youtube.com
Titration of a monoprotic strong acid (HCl) and monoprotic strong base Titration Hcl Naoh Berechnung Let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a strong base (1.0 m naoh). Determination of sodium hydroxide concentration is about as. Titrationskurve starke säure mit starker base. Hcl and naoh are strong acid and strong base respectively and their titration curves are similar (shape of curve) in different concentrations. Titration of sodium hydroxide. Titration Hcl Naoh Berechnung.
From www.chemistryscl.com
NaOH and HCl Titration Curves Selecting Indicators Titration Hcl Naoh Berechnung Berechne eine unbekannte konzentration von 0,15 ml hcl: Naoh + hcl = nacl + h 2 o. Using the formula c1v1 = c2v2, where c1 is the molarity of the naoh, the difference in. Titration of sodium hydroxide with hydrochloric acid. Determination of sodium hydroxide concentration is about as. Die titrationskurve einer starken säure mit einer starken base ergibt sich. Titration Hcl Naoh Berechnung.