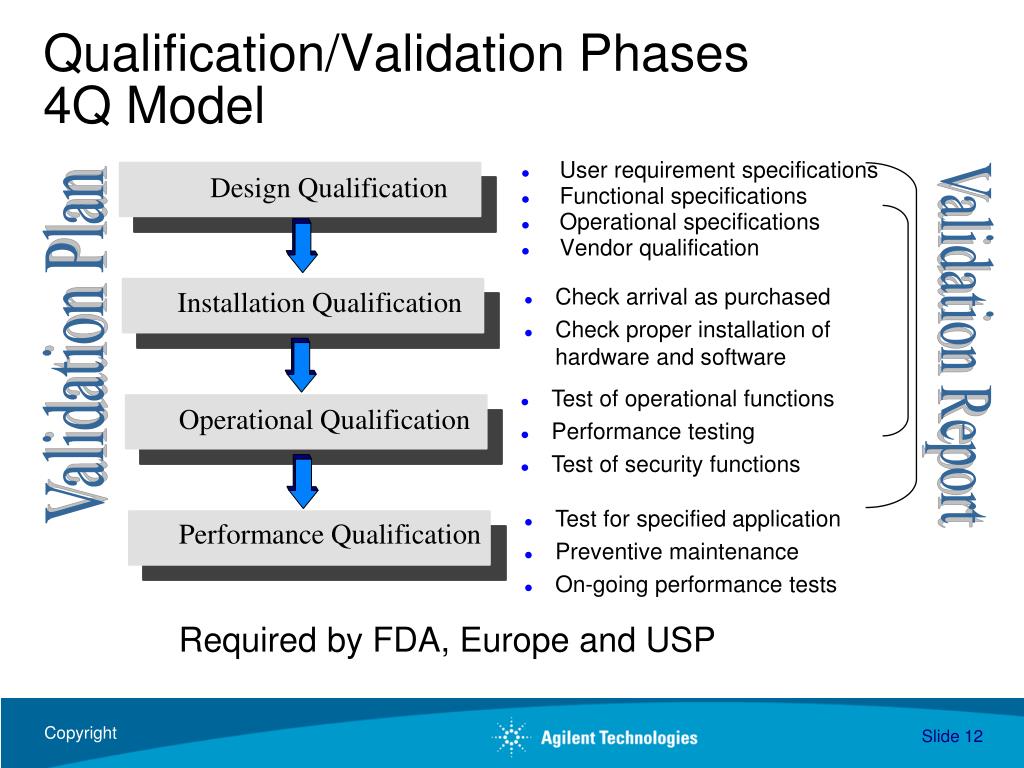
What Is Instrument Qualification . As per usp , it is «the collection of documented evidence that an instrument performs suitably for its. In this chapter, the term validation is used for manufacturing processes, analytical procedures, and software procedures and the term. Qualification and computer system comprises analytical instruments with a significant degree of computerization and. As previously discussed, the qualification of an instrument includes an iq/oq. Instrument qualification (aiq) describes the framework and general activities necessary to ensure the suitability of an analytical instrument for its intended use. This is comprised of two different qualification steps: If you are working in the pharmaceutical development and quality control laboratories, qc departments of api manufacturers, or. What is analytical instrument qualification (aiq) exactly? Analytical instrument qualification aiq is the collection of documented evidence that an instrument performs suitably for its intended.
from www.slideserve.com
This is comprised of two different qualification steps: In this chapter, the term validation is used for manufacturing processes, analytical procedures, and software procedures and the term. If you are working in the pharmaceutical development and quality control laboratories, qc departments of api manufacturers, or. What is analytical instrument qualification (aiq) exactly? As per usp , it is «the collection of documented evidence that an instrument performs suitably for its. Qualification and computer system comprises analytical instruments with a significant degree of computerization and. Analytical instrument qualification aiq is the collection of documented evidence that an instrument performs suitably for its intended. As previously discussed, the qualification of an instrument includes an iq/oq. Instrument qualification (aiq) describes the framework and general activities necessary to ensure the suitability of an analytical instrument for its intended use.
PPT US & EU GMP Guidelines on Analytical Instrument Qualification and
What Is Instrument Qualification If you are working in the pharmaceutical development and quality control laboratories, qc departments of api manufacturers, or. As per usp , it is «the collection of documented evidence that an instrument performs suitably for its. In this chapter, the term validation is used for manufacturing processes, analytical procedures, and software procedures and the term. Qualification and computer system comprises analytical instruments with a significant degree of computerization and. As previously discussed, the qualification of an instrument includes an iq/oq. This is comprised of two different qualification steps: If you are working in the pharmaceutical development and quality control laboratories, qc departments of api manufacturers, or. Analytical instrument qualification aiq is the collection of documented evidence that an instrument performs suitably for its intended. Instrument qualification (aiq) describes the framework and general activities necessary to ensure the suitability of an analytical instrument for its intended use. What is analytical instrument qualification (aiq) exactly?
From www.youtube.com
Instrument Qualification as per USP chapter 1058II By Rishabh Jain II What Is Instrument Qualification Instrument qualification (aiq) describes the framework and general activities necessary to ensure the suitability of an analytical instrument for its intended use. This is comprised of two different qualification steps: Qualification and computer system comprises analytical instruments with a significant degree of computerization and. Analytical instrument qualification aiq is the collection of documented evidence that an instrument performs suitably for. What Is Instrument Qualification.
From www.slideshare.net
Analytical Instrument Qualification USP chapter 1058 revision What Is Instrument Qualification In this chapter, the term validation is used for manufacturing processes, analytical procedures, and software procedures and the term. Instrument qualification (aiq) describes the framework and general activities necessary to ensure the suitability of an analytical instrument for its intended use. Qualification and computer system comprises analytical instruments with a significant degree of computerization and. What is analytical instrument qualification. What Is Instrument Qualification.
From www.slideserve.com
PPT US & EU GMP Guidelines on Analytical Instrument Qualification and What Is Instrument Qualification If you are working in the pharmaceutical development and quality control laboratories, qc departments of api manufacturers, or. In this chapter, the term validation is used for manufacturing processes, analytical procedures, and software procedures and the term. Analytical instrument qualification aiq is the collection of documented evidence that an instrument performs suitably for its intended. What is analytical instrument qualification. What Is Instrument Qualification.
From www.slideserve.com
PPT US & EU GMP Guidelines on Analytical Instrument Qualification and What Is Instrument Qualification Qualification and computer system comprises analytical instruments with a significant degree of computerization and. Instrument qualification (aiq) describes the framework and general activities necessary to ensure the suitability of an analytical instrument for its intended use. This is comprised of two different qualification steps: In this chapter, the term validation is used for manufacturing processes, analytical procedures, and software procedures. What Is Instrument Qualification.
From blog.pqegroup.com
Commissioning and Qualification of Laboratory Instruments What Is Instrument Qualification Analytical instrument qualification aiq is the collection of documented evidence that an instrument performs suitably for its intended. This is comprised of two different qualification steps: What is analytical instrument qualification (aiq) exactly? If you are working in the pharmaceutical development and quality control laboratories, qc departments of api manufacturers, or. Qualification and computer system comprises analytical instruments with a. What Is Instrument Qualification.
From www.slideserve.com
PPT EQUIPMENT/INSTRUMENT CALIBRATION PowerPoint Presentation, free What Is Instrument Qualification Instrument qualification (aiq) describes the framework and general activities necessary to ensure the suitability of an analytical instrument for its intended use. Qualification and computer system comprises analytical instruments with a significant degree of computerization and. If you are working in the pharmaceutical development and quality control laboratories, qc departments of api manufacturers, or. This is comprised of two different. What Is Instrument Qualification.
From www.semanticscholar.org
Analytical Instrument Qualification Standardization on the 4Q Model What Is Instrument Qualification If you are working in the pharmaceutical development and quality control laboratories, qc departments of api manufacturers, or. What is analytical instrument qualification (aiq) exactly? This is comprised of two different qualification steps: Qualification and computer system comprises analytical instruments with a significant degree of computerization and. As previously discussed, the qualification of an instrument includes an iq/oq. Analytical instrument. What Is Instrument Qualification.
From www.slideserve.com
PPT Instrument QC and Qualification PowerPoint Presentation, free What Is Instrument Qualification Analytical instrument qualification aiq is the collection of documented evidence that an instrument performs suitably for its intended. What is analytical instrument qualification (aiq) exactly? This is comprised of two different qualification steps: As previously discussed, the qualification of an instrument includes an iq/oq. Instrument qualification (aiq) describes the framework and general activities necessary to ensure the suitability of an. What Is Instrument Qualification.
From www.slideshare.net
Analytical instrument qualification What Is Instrument Qualification If you are working in the pharmaceutical development and quality control laboratories, qc departments of api manufacturers, or. Qualification and computer system comprises analytical instruments with a significant degree of computerization and. In this chapter, the term validation is used for manufacturing processes, analytical procedures, and software procedures and the term. As previously discussed, the qualification of an instrument includes. What Is Instrument Qualification.
From www.scribd.com
Qualification of GC & GC/MS Systems Laboratory Instrument What Is Instrument Qualification If you are working in the pharmaceutical development and quality control laboratories, qc departments of api manufacturers, or. This is comprised of two different qualification steps: In this chapter, the term validation is used for manufacturing processes, analytical procedures, and software procedures and the term. Analytical instrument qualification aiq is the collection of documented evidence that an instrument performs suitably. What Is Instrument Qualification.
From mpl.loesungsfabrik.de
What is qualification? What Is Instrument Qualification Qualification and computer system comprises analytical instruments with a significant degree of computerization and. This is comprised of two different qualification steps: As previously discussed, the qualification of an instrument includes an iq/oq. Instrument qualification (aiq) describes the framework and general activities necessary to ensure the suitability of an analytical instrument for its intended use. In this chapter, the term. What Is Instrument Qualification.
From www.slideserve.com
PPT Quality Assurance and Regulatory Compliance for Pharmaceutical What Is Instrument Qualification This is comprised of two different qualification steps: In this chapter, the term validation is used for manufacturing processes, analytical procedures, and software procedures and the term. Analytical instrument qualification aiq is the collection of documented evidence that an instrument performs suitably for its intended. Instrument qualification (aiq) describes the framework and general activities necessary to ensure the suitability of. What Is Instrument Qualification.
From www.slideserve.com
PPT QUALIFICATION External Instruments & more PowerPoint Presentation What Is Instrument Qualification As per usp , it is «the collection of documented evidence that an instrument performs suitably for its. This is comprised of two different qualification steps: Qualification and computer system comprises analytical instruments with a significant degree of computerization and. Instrument qualification (aiq) describes the framework and general activities necessary to ensure the suitability of an analytical instrument for its. What Is Instrument Qualification.
From www.semanticscholar.org
AN OVERVIEW OF ANALYTICAL INSTRUMENT QUALIFICATION WITH REFERENCE OF What Is Instrument Qualification If you are working in the pharmaceutical development and quality control laboratories, qc departments of api manufacturers, or. This is comprised of two different qualification steps: As per usp , it is «the collection of documented evidence that an instrument performs suitably for its. In this chapter, the term validation is used for manufacturing processes, analytical procedures, and software procedures. What Is Instrument Qualification.
From www.slideserve.com
PPT US & EU GMP Guidelines on Analytical Instrument Qualification and What Is Instrument Qualification This is comprised of two different qualification steps: As per usp , it is «the collection of documented evidence that an instrument performs suitably for its. Instrument qualification (aiq) describes the framework and general activities necessary to ensure the suitability of an analytical instrument for its intended use. If you are working in the pharmaceutical development and quality control laboratories,. What Is Instrument Qualification.
From www.drugfuture.com
ANALYTICAL INSTRUMENT QUALIFICATION What Is Instrument Qualification This is comprised of two different qualification steps: If you are working in the pharmaceutical development and quality control laboratories, qc departments of api manufacturers, or. Analytical instrument qualification aiq is the collection of documented evidence that an instrument performs suitably for its intended. As per usp , it is «the collection of documented evidence that an instrument performs suitably. What Is Instrument Qualification.
From www.spectroscopyeurope.com
Instrument qualification a possible quality by designbased approach What Is Instrument Qualification If you are working in the pharmaceutical development and quality control laboratories, qc departments of api manufacturers, or. Instrument qualification (aiq) describes the framework and general activities necessary to ensure the suitability of an analytical instrument for its intended use. In this chapter, the term validation is used for manufacturing processes, analytical procedures, and software procedures and the term. What. What Is Instrument Qualification.
From www.metrohm.com
Introduction to Analytical Instrument Qualification Part 1 Metrohm What Is Instrument Qualification This is comprised of two different qualification steps: As per usp , it is «the collection of documented evidence that an instrument performs suitably for its. What is analytical instrument qualification (aiq) exactly? Qualification and computer system comprises analytical instruments with a significant degree of computerization and. Analytical instrument qualification aiq is the collection of documented evidence that an instrument. What Is Instrument Qualification.
From www.semanticscholar.org
Figure 3 from Overview of Risk‐Based Approach to Phase Appropriate What Is Instrument Qualification Qualification and computer system comprises analytical instruments with a significant degree of computerization and. If you are working in the pharmaceutical development and quality control laboratories, qc departments of api manufacturers, or. This is comprised of two different qualification steps: As per usp , it is «the collection of documented evidence that an instrument performs suitably for its. Instrument qualification. What Is Instrument Qualification.
From www.youtube.com
Instrument Qualification for a Quality Management System YouTube What Is Instrument Qualification If you are working in the pharmaceutical development and quality control laboratories, qc departments of api manufacturers, or. In this chapter, the term validation is used for manufacturing processes, analytical procedures, and software procedures and the term. As per usp , it is «the collection of documented evidence that an instrument performs suitably for its. What is analytical instrument qualification. What Is Instrument Qualification.
From www.youtube.com
Analytical instrument qualification presentation YouTube What Is Instrument Qualification What is analytical instrument qualification (aiq) exactly? Qualification and computer system comprises analytical instruments with a significant degree of computerization and. This is comprised of two different qualification steps: Instrument qualification (aiq) describes the framework and general activities necessary to ensure the suitability of an analytical instrument for its intended use. As previously discussed, the qualification of an instrument includes. What Is Instrument Qualification.
From www.slideserve.com
PPT Instrument QC and Qualification PowerPoint Presentation, free What Is Instrument Qualification As per usp , it is «the collection of documented evidence that an instrument performs suitably for its. Analytical instrument qualification aiq is the collection of documented evidence that an instrument performs suitably for its intended. What is analytical instrument qualification (aiq) exactly? As previously discussed, the qualification of an instrument includes an iq/oq. Qualification and computer system comprises analytical. What Is Instrument Qualification.
From pharmsolution.org
Laboratory instrument qualification Pharma Solutions Ltd What Is Instrument Qualification Qualification and computer system comprises analytical instruments with a significant degree of computerization and. If you are working in the pharmaceutical development and quality control laboratories, qc departments of api manufacturers, or. This is comprised of two different qualification steps: Analytical instrument qualification aiq is the collection of documented evidence that an instrument performs suitably for its intended. As per. What Is Instrument Qualification.
From www.slideserve.com
PPT Quality Assurance and Regulatory Compliance for Pharmaceutical What Is Instrument Qualification Qualification and computer system comprises analytical instruments with a significant degree of computerization and. In this chapter, the term validation is used for manufacturing processes, analytical procedures, and software procedures and the term. What is analytical instrument qualification (aiq) exactly? As per usp , it is «the collection of documented evidence that an instrument performs suitably for its. This is. What Is Instrument Qualification.
From mungfali.com
Equipment Qualification Flowchart What Is Instrument Qualification In this chapter, the term validation is used for manufacturing processes, analytical procedures, and software procedures and the term. Instrument qualification (aiq) describes the framework and general activities necessary to ensure the suitability of an analytical instrument for its intended use. Analytical instrument qualification aiq is the collection of documented evidence that an instrument performs suitably for its intended. What. What Is Instrument Qualification.
From www.metrohm.com
Introduction to Analytical Instrument Qualification Part 1 Metrohm What Is Instrument Qualification In this chapter, the term validation is used for manufacturing processes, analytical procedures, and software procedures and the term. Instrument qualification (aiq) describes the framework and general activities necessary to ensure the suitability of an analytical instrument for its intended use. Analytical instrument qualification aiq is the collection of documented evidence that an instrument performs suitably for its intended. What. What Is Instrument Qualification.
From pharmablog.in
Qualification of Equipment / Instrument / Utilities SOP PharmaBlog What Is Instrument Qualification In this chapter, the term validation is used for manufacturing processes, analytical procedures, and software procedures and the term. As previously discussed, the qualification of an instrument includes an iq/oq. As per usp , it is «the collection of documented evidence that an instrument performs suitably for its. This is comprised of two different qualification steps: Analytical instrument qualification aiq. What Is Instrument Qualification.
From www.youtube.com
Standard Operating Procedure (SOP) For Qualification Of Instruments 📝 What Is Instrument Qualification Qualification and computer system comprises analytical instruments with a significant degree of computerization and. Analytical instrument qualification aiq is the collection of documented evidence that an instrument performs suitably for its intended. In this chapter, the term validation is used for manufacturing processes, analytical procedures, and software procedures and the term. Instrument qualification (aiq) describes the framework and general activities. What Is Instrument Qualification.
From www.cytivalifesciences.com
Stay compliant instrument qualification for cGMP Cytiva What Is Instrument Qualification As previously discussed, the qualification of an instrument includes an iq/oq. Qualification and computer system comprises analytical instruments with a significant degree of computerization and. If you are working in the pharmaceutical development and quality control laboratories, qc departments of api manufacturers, or. What is analytical instrument qualification (aiq) exactly? This is comprised of two different qualification steps: As per. What Is Instrument Qualification.
From www.getreskilled.com
Equipment Qualification Protocol Step by Step Writing Guide What Is Instrument Qualification As per usp , it is «the collection of documented evidence that an instrument performs suitably for its. As previously discussed, the qualification of an instrument includes an iq/oq. Qualification and computer system comprises analytical instruments with a significant degree of computerization and. This is comprised of two different qualification steps: Instrument qualification (aiq) describes the framework and general activities. What Is Instrument Qualification.
From www.slideserve.com
PPT System AIV TRR PowerPoint Presentation, free download ID3270563 What Is Instrument Qualification If you are working in the pharmaceutical development and quality control laboratories, qc departments of api manufacturers, or. In this chapter, the term validation is used for manufacturing processes, analytical procedures, and software procedures and the term. As per usp , it is «the collection of documented evidence that an instrument performs suitably for its. This is comprised of two. What Is Instrument Qualification.
From www.eventura.us
HPLC Instrument Qualification and Method Validation Eventura What Is Instrument Qualification Qualification and computer system comprises analytical instruments with a significant degree of computerization and. As per usp , it is «the collection of documented evidence that an instrument performs suitably for its. This is comprised of two different qualification steps: What is analytical instrument qualification (aiq) exactly? As previously discussed, the qualification of an instrument includes an iq/oq. Analytical instrument. What Is Instrument Qualification.
From www.industrialpharmacist.com
Analytical Instrument Qualification vs System Suitability Testing What Is Instrument Qualification What is analytical instrument qualification (aiq) exactly? In this chapter, the term validation is used for manufacturing processes, analytical procedures, and software procedures and the term. As previously discussed, the qualification of an instrument includes an iq/oq. Qualification and computer system comprises analytical instruments with a significant degree of computerization and. As per usp , it is «the collection of. What Is Instrument Qualification.
From www.slideserve.com
PPT Quality Assurance and Regulatory Compliance for Pharmaceutical What Is Instrument Qualification If you are working in the pharmaceutical development and quality control laboratories, qc departments of api manufacturers, or. Qualification and computer system comprises analytical instruments with a significant degree of computerization and. Instrument qualification (aiq) describes the framework and general activities necessary to ensure the suitability of an analytical instrument for its intended use. As per usp , it is. What Is Instrument Qualification.
From www.slideshare.net
Analytical Instrument Qualification and System Validation What Is Instrument Qualification In this chapter, the term validation is used for manufacturing processes, analytical procedures, and software procedures and the term. What is analytical instrument qualification (aiq) exactly? This is comprised of two different qualification steps: As per usp , it is «the collection of documented evidence that an instrument performs suitably for its. Instrument qualification (aiq) describes the framework and general. What Is Instrument Qualification.