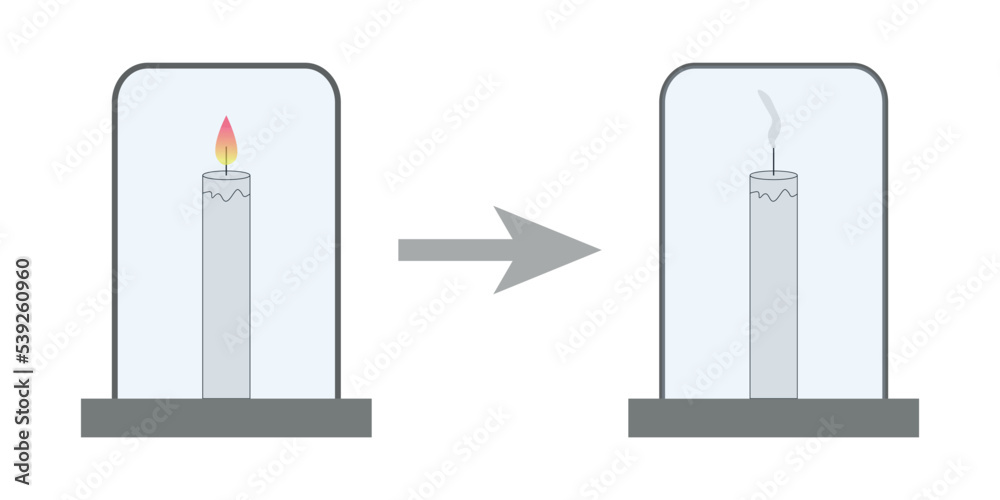
Oxygen Candle Equation . It comes from the air. Teach why fire needs oxygen with a simple candle under glass experiment. Oxygen exhibits many unique physical and chemical properties. The substance that reacts with the candle wax is oxygen. For example, oxygen is a colorless and odorless gas, with a density greater than that of air, and a very low solubility in water. Putting the jar over the candle keeps oxygen from outside the jar. Combustion of an element can be described using the following chemical equation: As wax is consumed, capillary action draws more liquid wax along the wick. Learn the chemical reactions when candle burns. These hydrocarbon molecules can burn completely. The heat of the flame vaporizes the wax molecules and they react with the oxygen in the air. Free elemental oxygen occurs naturally as a gas in the form of diatomic molecules, \(\ce{o2}\) (g). When you light a candle, wax near the wick melts into a liquid. When you trap the candle in a jar, it only has a limited amount of oxygen. Josh finds out that in larger.
from stock.adobe.com
These hydrocarbon molecules can burn completely. Learn the chemical reactions when candle burns. Putting the jar over the candle keeps oxygen from outside the jar. For example, oxygen is a colorless and odorless gas, with a density greater than that of air, and a very low solubility in water. It comes from the air. Oxygen exhibits many unique physical and chemical properties. Combustion of an element can be described using the following chemical equation: When you trap the candle in a jar, it only has a limited amount of oxygen. A candle flame is the result of a chemical reaction between wax gas and oxygen in the air. Teach why fire needs oxygen with a simple candle under glass experiment.
Candle science experiment diagram. Oxygen and fire experiment. Vector
Oxygen Candle Equation When you trap the candle in a jar, it only has a limited amount of oxygen. Putting the jar over the candle keeps oxygen from outside the jar. When you trap the candle in a jar, it only has a limited amount of oxygen. Free elemental oxygen occurs naturally as a gas in the form of diatomic molecules, \(\ce{o2}\) (g). The substance that reacts with the candle wax is oxygen. When you light a candle, wax near the wick melts into a liquid. As wax is consumed, capillary action draws more liquid wax along the wick. A candle flame is the result of a chemical reaction between wax gas and oxygen in the air. Oxygen exhibits many unique physical and chemical properties. Combustion of an element can be described using the following chemical equation: Learn the chemical reactions when candle burns. Josh finds out that in larger. It comes from the air. For example, oxygen is a colorless and odorless gas, with a density greater than that of air, and a very low solubility in water. The heat of the flame vaporizes the wax molecules and they react with the oxygen in the air. These hydrocarbon molecules can burn completely.
From slideplayer.com
In a combustion reaction, a candle burns using the oxygen in the air Oxygen Candle Equation The substance that reacts with the candle wax is oxygen. For example, oxygen is a colorless and odorless gas, with a density greater than that of air, and a very low solubility in water. A candle flame is the result of a chemical reaction between wax gas and oxygen in the air. Free elemental oxygen occurs naturally as a gas. Oxygen Candle Equation.
From www.youtube.com
Oxygen in air candle and cylinder method YouTube Oxygen Candle Equation Josh finds out that in larger. A candle flame is the result of a chemical reaction between wax gas and oxygen in the air. These hydrocarbon molecules can burn completely. As wax is consumed, capillary action draws more liquid wax along the wick. Combustion of an element can be described using the following chemical equation: Teach why fire needs oxygen. Oxygen Candle Equation.
From www.vecteezy.com
Oxygen is needed for burning a candle vector illustration 20240697 Oxygen Candle Equation The heat of the flame vaporizes the wax molecules and they react with the oxygen in the air. For example, oxygen is a colorless and odorless gas, with a density greater than that of air, and a very low solubility in water. Combustion of an element can be described using the following chemical equation: Oxygen exhibits many unique physical and. Oxygen Candle Equation.
From www.slideshare.net
simple chemical reactions chemistry Oxygen Candle Equation As wax is consumed, capillary action draws more liquid wax along the wick. When you light a candle, wax near the wick melts into a liquid. It comes from the air. These hydrocarbon molecules can burn completely. A candle flame is the result of a chemical reaction between wax gas and oxygen in the air. Combustion of an element can. Oxygen Candle Equation.
From www.numerade.com
SOLVED An oxygen candle is a cylindrical chemical oxygen generator Oxygen Candle Equation A candle flame is the result of a chemical reaction between wax gas and oxygen in the air. Teach why fire needs oxygen with a simple candle under glass experiment. Oxygen exhibits many unique physical and chemical properties. The heat of the flame vaporizes the wax molecules and they react with the oxygen in the air. These hydrocarbon molecules can. Oxygen Candle Equation.
From www.numerade.com
SOLVED Paraffin, a wax used to make candles, has a formula of C25H52 Oxygen Candle Equation Combustion of an element can be described using the following chemical equation: It comes from the air. The heat of the flame vaporizes the wax molecules and they react with the oxygen in the air. As wax is consumed, capillary action draws more liquid wax along the wick. Learn the chemical reactions when candle burns. For example, oxygen is a. Oxygen Candle Equation.
From mekelas.blogspot.com
Combustion Experiment With Candle / function Oxygen In the Combustion Oxygen Candle Equation Oxygen exhibits many unique physical and chemical properties. When you light a candle, wax near the wick melts into a liquid. Teach why fire needs oxygen with a simple candle under glass experiment. It comes from the air. Combustion of an element can be described using the following chemical equation: These hydrocarbon molecules can burn completely. As wax is consumed,. Oxygen Candle Equation.
From mekelas.blogspot.com
Combustion Experiment With Candle / function Oxygen In the Combustion Oxygen Candle Equation When you light a candle, wax near the wick melts into a liquid. The heat of the flame vaporizes the wax molecules and they react with the oxygen in the air. Free elemental oxygen occurs naturally as a gas in the form of diatomic molecules, \(\ce{o2}\) (g). Josh finds out that in larger. Putting the jar over the candle keeps. Oxygen Candle Equation.
From www.slideserve.com
PPT Equations & Reactions PowerPoint Presentation, free download ID Oxygen Candle Equation Free elemental oxygen occurs naturally as a gas in the form of diatomic molecules, \(\ce{o2}\) (g). The substance that reacts with the candle wax is oxygen. It comes from the air. When you trap the candle in a jar, it only has a limited amount of oxygen. For example, oxygen is a colorless and odorless gas, with a density greater. Oxygen Candle Equation.
From www.youtube.com
C25H52+O2=CO2+H20 Balanced EquationWax +Oxygen=Carbon dioxide+Water Oxygen Candle Equation For example, oxygen is a colorless and odorless gas, with a density greater than that of air, and a very low solubility in water. A candle flame is the result of a chemical reaction between wax gas and oxygen in the air. Oxygen exhibits many unique physical and chemical properties. The substance that reacts with the candle wax is oxygen.. Oxygen Candle Equation.
From www.researchgate.net
Schematic of heat transfer and oxygen release of solidstructured Oxygen Candle Equation Putting the jar over the candle keeps oxygen from outside the jar. Free elemental oxygen occurs naturally as a gas in the form of diatomic molecules, \(\ce{o2}\) (g). The heat of the flame vaporizes the wax molecules and they react with the oxygen in the air. Combustion of an element can be described using the following chemical equation: Josh finds. Oxygen Candle Equation.
From www.youtube.com
How to Balance C25H52 + O2 = CO2 + H2O (Wax + Oxygen gas ) YouTube Oxygen Candle Equation A candle flame is the result of a chemical reaction between wax gas and oxygen in the air. It comes from the air. Free elemental oxygen occurs naturally as a gas in the form of diatomic molecules, \(\ce{o2}\) (g). When you light a candle, wax near the wick melts into a liquid. These hydrocarbon molecules can burn completely. Combustion of. Oxygen Candle Equation.
From in.pinterest.com
Exothermic Reaction definition A heatreleasing chemical reaction Oxygen Candle Equation As wax is consumed, capillary action draws more liquid wax along the wick. A candle flame is the result of a chemical reaction between wax gas and oxygen in the air. It comes from the air. These hydrocarbon molecules can burn completely. Putting the jar over the candle keeps oxygen from outside the jar. Combustion of an element can be. Oxygen Candle Equation.
From www.chegg.com
Solved An oxygen candle is a cylindrical chemical oxygen Oxygen Candle Equation For example, oxygen is a colorless and odorless gas, with a density greater than that of air, and a very low solubility in water. When you light a candle, wax near the wick melts into a liquid. Free elemental oxygen occurs naturally as a gas in the form of diatomic molecules, \(\ce{o2}\) (g). Josh finds out that in larger. These. Oxygen Candle Equation.
From www.alamy.com
Diagram of oxygen and fire experiment illustration Stock Vector Image Oxygen Candle Equation Free elemental oxygen occurs naturally as a gas in the form of diatomic molecules, \(\ce{o2}\) (g). These hydrocarbon molecules can burn completely. For example, oxygen is a colorless and odorless gas, with a density greater than that of air, and a very low solubility in water. Putting the jar over the candle keeps oxygen from outside the jar. It comes. Oxygen Candle Equation.
From www.youtube.com
How much oxygen does a candle use up? YouTube Oxygen Candle Equation The substance that reacts with the candle wax is oxygen. As wax is consumed, capillary action draws more liquid wax along the wick. A candle flame is the result of a chemical reaction between wax gas and oxygen in the air. For example, oxygen is a colorless and odorless gas, with a density greater than that of air, and a. Oxygen Candle Equation.
From peshkin.mech.northwestern.edu
Science Fair Project how much of our atmosphere is oxygen? Oxygen Candle Equation It comes from the air. Putting the jar over the candle keeps oxygen from outside the jar. When you trap the candle in a jar, it only has a limited amount of oxygen. Combustion of an element can be described using the following chemical equation: Free elemental oxygen occurs naturally as a gas in the form of diatomic molecules, \(\ce{o2}\). Oxygen Candle Equation.
From www.vecteezy.com
Oxygen is needed for burning a candle 23452917 Vector Art at Vecteezy Oxygen Candle Equation Josh finds out that in larger. The substance that reacts with the candle wax is oxygen. Free elemental oxygen occurs naturally as a gas in the form of diatomic molecules, \(\ce{o2}\) (g). Putting the jar over the candle keeps oxygen from outside the jar. Combustion of an element can be described using the following chemical equation: A candle flame is. Oxygen Candle Equation.
From www.youtube.com
Candle oxygen Experiment II School Science Projects II YouTube Oxygen Candle Equation These hydrocarbon molecules can burn completely. It comes from the air. Oxygen exhibits many unique physical and chemical properties. When you light a candle, wax near the wick melts into a liquid. Free elemental oxygen occurs naturally as a gas in the form of diatomic molecules, \(\ce{o2}\) (g). The substance that reacts with the candle wax is oxygen. Learn the. Oxygen Candle Equation.
From www.vectorstock.com
Fire and oxygen experiment with two candles Vector Image Oxygen Candle Equation For example, oxygen is a colorless and odorless gas, with a density greater than that of air, and a very low solubility in water. The heat of the flame vaporizes the wax molecules and they react with the oxygen in the air. Free elemental oxygen occurs naturally as a gas in the form of diatomic molecules, \(\ce{o2}\) (g). Learn the. Oxygen Candle Equation.
From www.numerade.com
SOLVED PreLab Assignment Using "The Law of Conservation of Mass Oxygen Candle Equation Combustion of an element can be described using the following chemical equation: Oxygen exhibits many unique physical and chemical properties. For example, oxygen is a colorless and odorless gas, with a density greater than that of air, and a very low solubility in water. When you light a candle, wax near the wick melts into a liquid. It comes from. Oxygen Candle Equation.
From vhmsscience8.weebly.com
Candle Lab VISTA HEIGHTS 8TH GRADE SCIENCE Oxygen Candle Equation These hydrocarbon molecules can burn completely. Learn the chemical reactions when candle burns. The heat of the flame vaporizes the wax molecules and they react with the oxygen in the air. Teach why fire needs oxygen with a simple candle under glass experiment. For example, oxygen is a colorless and odorless gas, with a density greater than that of air,. Oxygen Candle Equation.
From www.numerade.com
SOLVED Suppose the candle was placed in a large, sealed jar that Oxygen Candle Equation Free elemental oxygen occurs naturally as a gas in the form of diatomic molecules, \(\ce{o2}\) (g). When you light a candle, wax near the wick melts into a liquid. These hydrocarbon molecules can burn completely. Learn the chemical reactions when candle burns. Oxygen exhibits many unique physical and chemical properties. The heat of the flame vaporizes the wax molecules and. Oxygen Candle Equation.
From www.numerade.com
SOLVED PreLab Assignment Using "The Law of Conservation of Mass Oxygen Candle Equation Josh finds out that in larger. The heat of the flame vaporizes the wax molecules and they react with the oxygen in the air. Oxygen exhibits many unique physical and chemical properties. The substance that reacts with the candle wax is oxygen. Free elemental oxygen occurs naturally as a gas in the form of diatomic molecules, \(\ce{o2}\) (g). For example,. Oxygen Candle Equation.
From www.slideserve.com
PPT UNIT 1 FIRE DYNAMICS PowerPoint Presentation, free download ID Oxygen Candle Equation A candle flame is the result of a chemical reaction between wax gas and oxygen in the air. Josh finds out that in larger. It comes from the air. The substance that reacts with the candle wax is oxygen. Oxygen exhibits many unique physical and chemical properties. As wax is consumed, capillary action draws more liquid wax along the wick.. Oxygen Candle Equation.
From www.ck12.org
Oxygen, Carbon Dioxide, and Energy CK12 Foundation Oxygen Candle Equation A candle flame is the result of a chemical reaction between wax gas and oxygen in the air. Combustion of an element can be described using the following chemical equation: Josh finds out that in larger. Oxygen exhibits many unique physical and chemical properties. Learn the chemical reactions when candle burns. Putting the jar over the candle keeps oxygen from. Oxygen Candle Equation.
From www.youtube.com
Candle oxygen experiment YouTube Oxygen Candle Equation As wax is consumed, capillary action draws more liquid wax along the wick. When you trap the candle in a jar, it only has a limited amount of oxygen. Josh finds out that in larger. It comes from the air. When you light a candle, wax near the wick melts into a liquid. These hydrocarbon molecules can burn completely. Putting. Oxygen Candle Equation.
From www.youtube.com
How does oxygen affect burning candles? YouTube Oxygen Candle Equation Josh finds out that in larger. It comes from the air. These hydrocarbon molecules can burn completely. The substance that reacts with the candle wax is oxygen. When you light a candle, wax near the wick melts into a liquid. For example, oxygen is a colorless and odorless gas, with a density greater than that of air, and a very. Oxygen Candle Equation.
From unbelievable-facts.com
What is an Oxygen Candle? Oxygen Candle Equation When you light a candle, wax near the wick melts into a liquid. As wax is consumed, capillary action draws more liquid wax along the wick. Teach why fire needs oxygen with a simple candle under glass experiment. Free elemental oxygen occurs naturally as a gas in the form of diatomic molecules, \(\ce{o2}\) (g). A candle flame is the result. Oxygen Candle Equation.
From www.ingridscience.ca
Candle chemistry (wax combustion) ingridscience.ca Oxygen Candle Equation Combustion of an element can be described using the following chemical equation: When you light a candle, wax near the wick melts into a liquid. As wax is consumed, capillary action draws more liquid wax along the wick. Josh finds out that in larger. The substance that reacts with the candle wax is oxygen. Oxygen exhibits many unique physical and. Oxygen Candle Equation.
From tutorbin.com
Solved An oxygen candle is a cylindrical chemical oxygen generator Oxygen Candle Equation The heat of the flame vaporizes the wax molecules and they react with the oxygen in the air. Oxygen exhibits many unique physical and chemical properties. Learn the chemical reactions when candle burns. It comes from the air. When you trap the candle in a jar, it only has a limited amount of oxygen. As wax is consumed, capillary action. Oxygen Candle Equation.
From stock.adobe.com
Candle science experiment diagram. Oxygen and fire experiment. Vector Oxygen Candle Equation Josh finds out that in larger. When you light a candle, wax near the wick melts into a liquid. The heat of the flame vaporizes the wax molecules and they react with the oxygen in the air. Teach why fire needs oxygen with a simple candle under glass experiment. A candle flame is the result of a chemical reaction between. Oxygen Candle Equation.
From slideplayer.com
Title Reacting Metals with Oxygen ppt download Oxygen Candle Equation Putting the jar over the candle keeps oxygen from outside the jar. As wax is consumed, capillary action draws more liquid wax along the wick. The substance that reacts with the candle wax is oxygen. These hydrocarbon molecules can burn completely. For example, oxygen is a colorless and odorless gas, with a density greater than that of air, and a. Oxygen Candle Equation.
From fphoto.photoshelter.com
science chemistry oxidation reaction candle burning oxygen Oxygen Candle Equation As wax is consumed, capillary action draws more liquid wax along the wick. Putting the jar over the candle keeps oxygen from outside the jar. Combustion of an element can be described using the following chemical equation: Learn the chemical reactions when candle burns. When you light a candle, wax near the wick melts into a liquid. For example, oxygen. Oxygen Candle Equation.
From www.dreamstime.com
Fire and Oxygen. Experiment with Two Candles and Glass. Burning and Oxygen Candle Equation Free elemental oxygen occurs naturally as a gas in the form of diatomic molecules, \(\ce{o2}\) (g). The substance that reacts with the candle wax is oxygen. A candle flame is the result of a chemical reaction between wax gas and oxygen in the air. Teach why fire needs oxygen with a simple candle under glass experiment. Combustion of an element. Oxygen Candle Equation.