Data Table 1 Naoh Titration Volume . For our first titration curve, let’s consider the titration of 50.0 ml of 0.100 m hcl using a titrant of 0.200 m naoh. Determining the concentration of acetic acid data table 1. Step 10 titrate these samples and. When a strong base and a. Determine the molarity of oxalic acid solution that you prepared. Weigh out two more samples of your unknown, increasing or decreasing the mass to have the volume of naoh used be around 25ml. Be sure to report values with the correct number of significant figures. Complete the table by calculating the volume of naoh needed to complete the titration. To start the analysis for the concentration and percentage of acetic acid in vinegar using the given data, calculate the number of moles of. Naoh titration volume initial naoh volume (ml) final naoh volume (ml) total volume of naoh. From the volume dispensed (5.00 ml) and concentration of. Initial naoh volume (ml) final naoh volume (ml) total volume of naoh
from www.chegg.com
Naoh titration volume initial naoh volume (ml) final naoh volume (ml) total volume of naoh. Be sure to report values with the correct number of significant figures. To start the analysis for the concentration and percentage of acetic acid in vinegar using the given data, calculate the number of moles of. Determine the molarity of oxalic acid solution that you prepared. Determining the concentration of acetic acid data table 1. Step 10 titrate these samples and. From the volume dispensed (5.00 ml) and concentration of. Complete the table by calculating the volume of naoh needed to complete the titration. When a strong base and a. Initial naoh volume (ml) final naoh volume (ml) total volume of naoh
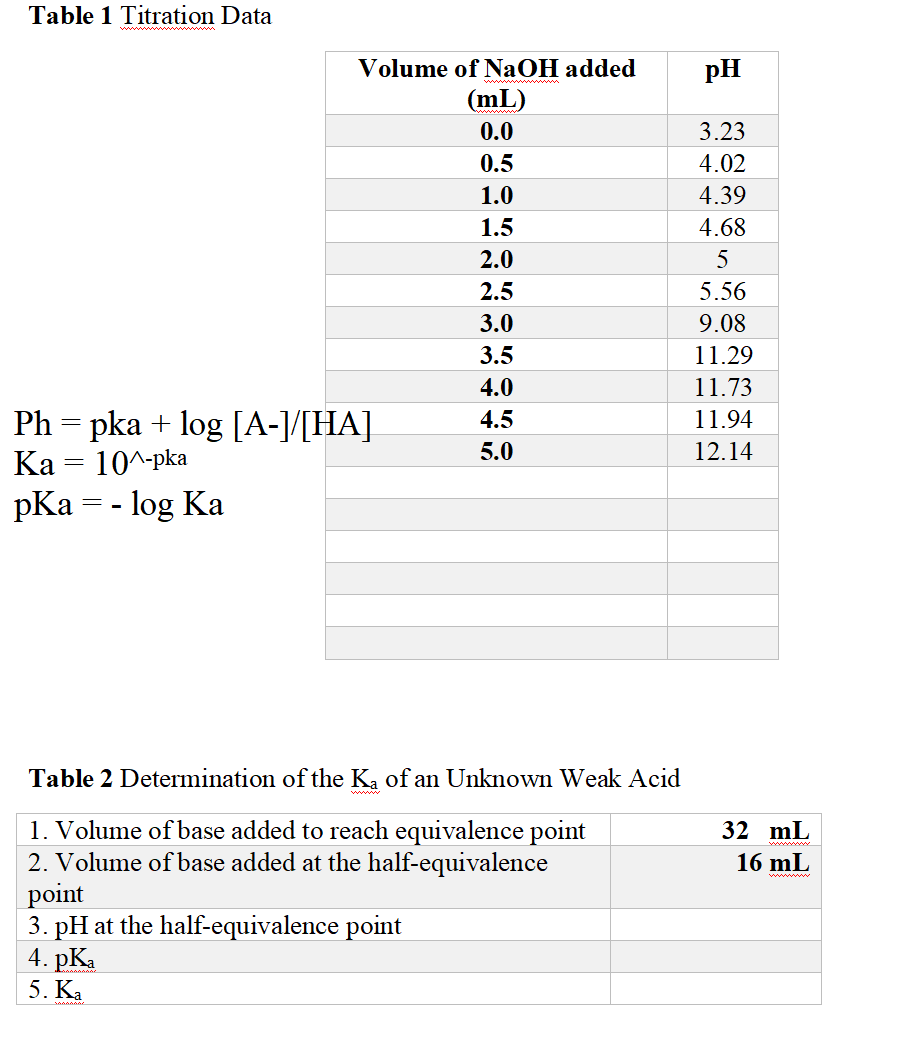
Solved Table 1 Titration Data pH Volume of NaOH added (mL)
Data Table 1 Naoh Titration Volume Naoh titration volume initial naoh volume (ml) final naoh volume (ml) total volume of naoh. Step 10 titrate these samples and. Determining the concentration of acetic acid data table 1. When a strong base and a. To start the analysis for the concentration and percentage of acetic acid in vinegar using the given data, calculate the number of moles of. Initial naoh volume (ml) final naoh volume (ml) total volume of naoh For our first titration curve, let’s consider the titration of 50.0 ml of 0.100 m hcl using a titrant of 0.200 m naoh. Complete the table by calculating the volume of naoh needed to complete the titration. Determine the molarity of oxalic acid solution that you prepared. Be sure to report values with the correct number of significant figures. Naoh titration volume initial naoh volume (ml) final naoh volume (ml) total volume of naoh. From the volume dispensed (5.00 ml) and concentration of. Weigh out two more samples of your unknown, increasing or decreasing the mass to have the volume of naoh used be around 25ml.
From www.researchgate.net
NaOH concentration, titration volume and pH value Download Table Data Table 1 Naoh Titration Volume From the volume dispensed (5.00 ml) and concentration of. When a strong base and a. Determine the molarity of oxalic acid solution that you prepared. Be sure to report values with the correct number of significant figures. Naoh titration volume initial naoh volume (ml) final naoh volume (ml) total volume of naoh. Weigh out two more samples of your unknown,. Data Table 1 Naoh Titration Volume.
From www.chegg.com
Solved Data Table 1 Titration of Vinegar with NaOH Vinegar Data Table 1 Naoh Titration Volume Be sure to report values with the correct number of significant figures. Weigh out two more samples of your unknown, increasing or decreasing the mass to have the volume of naoh used be around 25ml. To start the analysis for the concentration and percentage of acetic acid in vinegar using the given data, calculate the number of moles of. For. Data Table 1 Naoh Titration Volume.
From www.numerade.com
SOLVED Experiment 1 AcidBase Titration Standardization of NaOH Data Table 1 Naoh Titration Volume Naoh titration volume initial naoh volume (ml) final naoh volume (ml) total volume of naoh. Step 10 titrate these samples and. Determining the concentration of acetic acid data table 1. For our first titration curve, let’s consider the titration of 50.0 ml of 0.100 m hcl using a titrant of 0.200 m naoh. When a strong base and a. To. Data Table 1 Naoh Titration Volume.
From www.chegg.com
Solved Table 1. Measurements for standardization of NaOH Data Table 1 Naoh Titration Volume Complete the table by calculating the volume of naoh needed to complete the titration. Be sure to report values with the correct number of significant figures. Step 10 titrate these samples and. Weigh out two more samples of your unknown, increasing or decreasing the mass to have the volume of naoh used be around 25ml. From the volume dispensed (5.00. Data Table 1 Naoh Titration Volume.
From courses.lumenlearning.com
14.8 AcidBase Titrations General College Chemistry II Data Table 1 Naoh Titration Volume When a strong base and a. Weigh out two more samples of your unknown, increasing or decreasing the mass to have the volume of naoh used be around 25ml. Complete the table by calculating the volume of naoh needed to complete the titration. Determining the concentration of acetic acid data table 1. Naoh titration volume initial naoh volume (ml) final. Data Table 1 Naoh Titration Volume.
From www.chegg.com
Solved Data Table 1 NaOH Titration Volume Initial NaOH Data Table 1 Naoh Titration Volume Weigh out two more samples of your unknown, increasing or decreasing the mass to have the volume of naoh used be around 25ml. From the volume dispensed (5.00 ml) and concentration of. When a strong base and a. Naoh titration volume initial naoh volume (ml) final naoh volume (ml) total volume of naoh. Determine the molarity of oxalic acid solution. Data Table 1 Naoh Titration Volume.
From www.chegg.com
Solved Data Table 1 NaOH Titration Volume Initial NaOH Data Table 1 Naoh Titration Volume Be sure to report values with the correct number of significant figures. Complete the table by calculating the volume of naoh needed to complete the titration. For our first titration curve, let’s consider the titration of 50.0 ml of 0.100 m hcl using a titrant of 0.200 m naoh. Determine the molarity of oxalic acid solution that you prepared. Initial. Data Table 1 Naoh Titration Volume.
From www.numerade.com
SOLVED Table Titration Data for Virtual Titration between NaOH and Data Table 1 Naoh Titration Volume Determining the concentration of acetic acid data table 1. Weigh out two more samples of your unknown, increasing or decreasing the mass to have the volume of naoh used be around 25ml. Initial naoh volume (ml) final naoh volume (ml) total volume of naoh For our first titration curve, let’s consider the titration of 50.0 ml of 0.100 m hcl. Data Table 1 Naoh Titration Volume.
From www.chegg.com
Solved Data Table 2. Titration Curve values. Drops NaOH Data Table 1 Naoh Titration Volume From the volume dispensed (5.00 ml) and concentration of. Step 10 titrate these samples and. Complete the table by calculating the volume of naoh needed to complete the titration. When a strong base and a. Determining the concentration of acetic acid data table 1. Initial naoh volume (ml) final naoh volume (ml) total volume of naoh Determine the molarity of. Data Table 1 Naoh Titration Volume.
From www.chegg.com
Solved A Student Collected Their Titration Data For The S... Data Table 1 Naoh Titration Volume Be sure to report values with the correct number of significant figures. Weigh out two more samples of your unknown, increasing or decreasing the mass to have the volume of naoh used be around 25ml. Determine the molarity of oxalic acid solution that you prepared. Complete the table by calculating the volume of naoh needed to complete the titration. Initial. Data Table 1 Naoh Titration Volume.
From www.chegg.com
Data Table 1 Titration of White Vinegar with Sodium Data Table 1 Naoh Titration Volume Be sure to report values with the correct number of significant figures. When a strong base and a. Step 10 titrate these samples and. Complete the table by calculating the volume of naoh needed to complete the titration. To start the analysis for the concentration and percentage of acetic acid in vinegar using the given data, calculate the number of. Data Table 1 Naoh Titration Volume.
From www.chegg.com
Experiment 1 Data Table 1 NaOH Titration Data Table 1 Naoh Titration Volume Determining the concentration of acetic acid data table 1. Be sure to report values with the correct number of significant figures. When a strong base and a. Naoh titration volume initial naoh volume (ml) final naoh volume (ml) total volume of naoh. Determine the molarity of oxalic acid solution that you prepared. Initial naoh volume (ml) final naoh volume (ml). Data Table 1 Naoh Titration Volume.
From www.numerade.com
SOLVED Data Tables Titration of Acetic Acid Trial 2 Trial 3 Data Table 1 Naoh Titration Volume Weigh out two more samples of your unknown, increasing or decreasing the mass to have the volume of naoh used be around 25ml. Be sure to report values with the correct number of significant figures. For our first titration curve, let’s consider the titration of 50.0 ml of 0.100 m hcl using a titrant of 0.200 m naoh. Determining the. Data Table 1 Naoh Titration Volume.
From brainly.com
Record the final buret volume of NaOH titrant in the data table. Values Data Table 1 Naoh Titration Volume To start the analysis for the concentration and percentage of acetic acid in vinegar using the given data, calculate the number of moles of. Weigh out two more samples of your unknown, increasing or decreasing the mass to have the volume of naoh used be around 25ml. Naoh titration volume initial naoh volume (ml) final naoh volume (ml) total volume. Data Table 1 Naoh Titration Volume.
From chem4three.blogspot.com
CHEMISTRY 11 TITRATIONS Data Table 1 Naoh Titration Volume Weigh out two more samples of your unknown, increasing or decreasing the mass to have the volume of naoh used be around 25ml. When a strong base and a. From the volume dispensed (5.00 ml) and concentration of. Initial naoh volume (ml) final naoh volume (ml) total volume of naoh For our first titration curve, let’s consider the titration of. Data Table 1 Naoh Titration Volume.
From www.numerade.com
Data Table 1 NaOH Titration Volume Initial NaOH Volume (mL) Final NaOH Data Table 1 Naoh Titration Volume For our first titration curve, let’s consider the titration of 50.0 ml of 0.100 m hcl using a titrant of 0.200 m naoh. When a strong base and a. Weigh out two more samples of your unknown, increasing or decreasing the mass to have the volume of naoh used be around 25ml. To start the analysis for the concentration and. Data Table 1 Naoh Titration Volume.
From www.numerade.com
SOLVED (16pts Part A Determining the End Point Using Phenolphthalein Data Table 1 Naoh Titration Volume Weigh out two more samples of your unknown, increasing or decreasing the mass to have the volume of naoh used be around 25ml. To start the analysis for the concentration and percentage of acetic acid in vinegar using the given data, calculate the number of moles of. Determine the molarity of oxalic acid solution that you prepared. Be sure to. Data Table 1 Naoh Titration Volume.
From www.vrogue.co
Solved Acid Base Titrations Consider The Titration Of vrogue.co Data Table 1 Naoh Titration Volume For our first titration curve, let’s consider the titration of 50.0 ml of 0.100 m hcl using a titrant of 0.200 m naoh. Weigh out two more samples of your unknown, increasing or decreasing the mass to have the volume of naoh used be around 25ml. Determine the molarity of oxalic acid solution that you prepared. Complete the table by. Data Table 1 Naoh Titration Volume.
From www.vrogue.co
Titration Data Sheetmethod 1 Titration With Buret An vrogue.co Data Table 1 Naoh Titration Volume To start the analysis for the concentration and percentage of acetic acid in vinegar using the given data, calculate the number of moles of. For our first titration curve, let’s consider the titration of 50.0 ml of 0.100 m hcl using a titrant of 0.200 m naoh. Determine the molarity of oxalic acid solution that you prepared. Step 10 titrate. Data Table 1 Naoh Titration Volume.
From www.numerade.com
SOLVEDName Date Experiment 1l AcidBase Titration Data Table 1 Naoh Titration Volume Complete the table by calculating the volume of naoh needed to complete the titration. Weigh out two more samples of your unknown, increasing or decreasing the mass to have the volume of naoh used be around 25ml. Be sure to report values with the correct number of significant figures. Step 10 titrate these samples and. To start the analysis for. Data Table 1 Naoh Titration Volume.
From www.vrogue.co
Titration Data Sheetmethod 1 Titration With Buret An vrogue.co Data Table 1 Naoh Titration Volume Naoh titration volume initial naoh volume (ml) final naoh volume (ml) total volume of naoh. When a strong base and a. From the volume dispensed (5.00 ml) and concentration of. Step 10 titrate these samples and. Be sure to report values with the correct number of significant figures. Determining the concentration of acetic acid data table 1. Weigh out two. Data Table 1 Naoh Titration Volume.
From www.numerade.com
Titration for Acetic Acid in Vinegar Lab Report Exercise 1 Data Table 1 Naoh Titration Volume For our first titration curve, let’s consider the titration of 50.0 ml of 0.100 m hcl using a titrant of 0.200 m naoh. Step 10 titrate these samples and. Be sure to report values with the correct number of significant figures. Determining the concentration of acetic acid data table 1. When a strong base and a. To start the analysis. Data Table 1 Naoh Titration Volume.
From www.chegg.com
Solved Data Table 1 Titration of Vinegar with Sodium Data Table 1 Naoh Titration Volume Initial naoh volume (ml) final naoh volume (ml) total volume of naoh For our first titration curve, let’s consider the titration of 50.0 ml of 0.100 m hcl using a titrant of 0.200 m naoh. Determine the molarity of oxalic acid solution that you prepared. Step 10 titrate these samples and. Be sure to report values with the correct number. Data Table 1 Naoh Titration Volume.
From www.chegg.com
Solved Table 1 Titration Data pH Volume of NaOH added (mL) Data Table 1 Naoh Titration Volume Determining the concentration of acetic acid data table 1. Weigh out two more samples of your unknown, increasing or decreasing the mass to have the volume of naoh used be around 25ml. When a strong base and a. Be sure to report values with the correct number of significant figures. For our first titration curve, let’s consider the titration of. Data Table 1 Naoh Titration Volume.
From www.numerade.com
SOLVED lab kit Data Experiment Exercise Data Table Data Table 2 Data Data Table 1 Naoh Titration Volume To start the analysis for the concentration and percentage of acetic acid in vinegar using the given data, calculate the number of moles of. Be sure to report values with the correct number of significant figures. Weigh out two more samples of your unknown, increasing or decreasing the mass to have the volume of naoh used be around 25ml. Initial. Data Table 1 Naoh Titration Volume.
From www.chegg.com
Solved Data Table 1 NaOH Titration Volume\table[[,Initial Data Table 1 Naoh Titration Volume Naoh titration volume initial naoh volume (ml) final naoh volume (ml) total volume of naoh. Weigh out two more samples of your unknown, increasing or decreasing the mass to have the volume of naoh used be around 25ml. For our first titration curve, let’s consider the titration of 50.0 ml of 0.100 m hcl using a titrant of 0.200 m. Data Table 1 Naoh Titration Volume.
From www.chegg.com
Solved AcidBase Titration Data Tables NaOH concentration Data Table 1 Naoh Titration Volume Naoh titration volume initial naoh volume (ml) final naoh volume (ml) total volume of naoh. Determine the molarity of oxalic acid solution that you prepared. Determining the concentration of acetic acid data table 1. From the volume dispensed (5.00 ml) and concentration of. Initial naoh volume (ml) final naoh volume (ml) total volume of naoh Weigh out two more samples. Data Table 1 Naoh Titration Volume.
From www.chegg.com
Titration of Acetic Acid Data Table 1 NaOH Titration Data Table 1 Naoh Titration Volume Determining the concentration of acetic acid data table 1. Step 10 titrate these samples and. From the volume dispensed (5.00 ml) and concentration of. Be sure to report values with the correct number of significant figures. Initial naoh volume (ml) final naoh volume (ml) total volume of naoh Determine the molarity of oxalic acid solution that you prepared. To start. Data Table 1 Naoh Titration Volume.
From www.chegg.com
Solved Summary Data Table AcidBase Titration CHEZ 101L Data Table 1 Naoh Titration Volume Naoh titration volume initial naoh volume (ml) final naoh volume (ml) total volume of naoh. When a strong base and a. Be sure to report values with the correct number of significant figures. Weigh out two more samples of your unknown, increasing or decreasing the mass to have the volume of naoh used be around 25ml. Determining the concentration of. Data Table 1 Naoh Titration Volume.
From www.chegg.com
Solved Data Table I Standardization of a NaOH Solution (0.5 Data Table 1 Naoh Titration Volume Determining the concentration of acetic acid data table 1. Weigh out two more samples of your unknown, increasing or decreasing the mass to have the volume of naoh used be around 25ml. Be sure to report values with the correct number of significant figures. Determine the molarity of oxalic acid solution that you prepared. To start the analysis for the. Data Table 1 Naoh Titration Volume.
From www.chegg.com
Solved Data Table 1 NaOH Titration Volume Initial NaOH Data Table 1 Naoh Titration Volume Naoh titration volume initial naoh volume (ml) final naoh volume (ml) total volume of naoh. To start the analysis for the concentration and percentage of acetic acid in vinegar using the given data, calculate the number of moles of. Determining the concentration of acetic acid data table 1. For our first titration curve, let’s consider the titration of 50.0 ml. Data Table 1 Naoh Titration Volume.
From www.chegg.com
Solved Data Table 1 NaOH Titration Volume Initial NaOH Data Table 1 Naoh Titration Volume Complete the table by calculating the volume of naoh needed to complete the titration. Weigh out two more samples of your unknown, increasing or decreasing the mass to have the volume of naoh used be around 25ml. Naoh titration volume initial naoh volume (ml) final naoh volume (ml) total volume of naoh. For our first titration curve, let’s consider the. Data Table 1 Naoh Titration Volume.
From www.numerade.com
SOLVED LABORATORY REPORT Determination of acetic acid in vinegar by Data Table 1 Naoh Titration Volume Initial naoh volume (ml) final naoh volume (ml) total volume of naoh Be sure to report values with the correct number of significant figures. Step 10 titrate these samples and. For our first titration curve, let’s consider the titration of 50.0 ml of 0.100 m hcl using a titrant of 0.200 m naoh. Complete the table by calculating the volume. Data Table 1 Naoh Titration Volume.
From www.chegg.com
Solved Data Table 1 NaOH Titration Volume Initial NaOH Data Table 1 Naoh Titration Volume Weigh out two more samples of your unknown, increasing or decreasing the mass to have the volume of naoh used be around 25ml. Initial naoh volume (ml) final naoh volume (ml) total volume of naoh Step 10 titrate these samples and. Determining the concentration of acetic acid data table 1. To start the analysis for the concentration and percentage of. Data Table 1 Naoh Titration Volume.
From www.chegg.com
Solved Below is a graph of pH vs. volume of NaOH(mL) added Data Table 1 Naoh Titration Volume Weigh out two more samples of your unknown, increasing or decreasing the mass to have the volume of naoh used be around 25ml. Determine the molarity of oxalic acid solution that you prepared. From the volume dispensed (5.00 ml) and concentration of. Determining the concentration of acetic acid data table 1. Be sure to report values with the correct number. Data Table 1 Naoh Titration Volume.