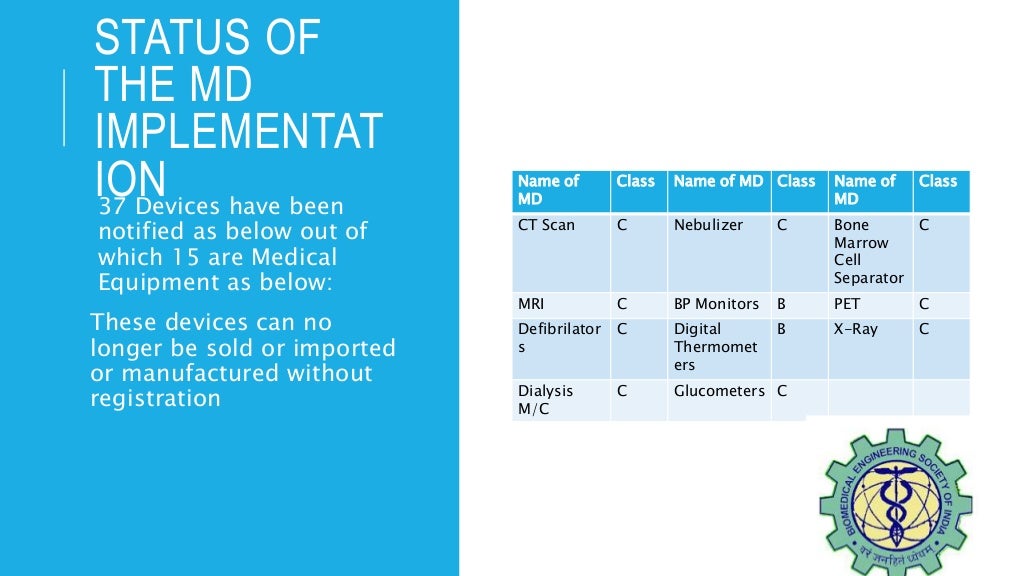
Medical Devices Rules 2017 . The medical device rules, 2017 asad ullah legislation, sros november 7, 2021 november 7, 2022. Find the latest circulars, notifications and amendments related to the medical devices rules, 2017 issued by the central drugs. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. A comprehensive overview of the new regulatory framework for medical devices in india, effective from january 1, 2018. The medical devices rules, 2017 are applicable to substances and devices notified under the drugs and cosmetics act, 1940. This document provides information on the regulatory requirements, quality management systems and standards for medical devices in.
from www.slideshare.net
Find the latest circulars, notifications and amendments related to the medical devices rules, 2017 issued by the central drugs. This document provides information on the regulatory requirements, quality management systems and standards for medical devices in. The medical device rules, 2017 asad ullah legislation, sros november 7, 2021 november 7, 2022. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. The medical devices rules, 2017 are applicable to substances and devices notified under the drugs and cosmetics act, 1940. A comprehensive overview of the new regulatory framework for medical devices in india, effective from january 1, 2018.
Medical Devices Rules 2017 Implementation
Medical Devices Rules 2017 Find the latest circulars, notifications and amendments related to the medical devices rules, 2017 issued by the central drugs. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. The medical device rules, 2017 asad ullah legislation, sros november 7, 2021 november 7, 2022. This document provides information on the regulatory requirements, quality management systems and standards for medical devices in. The medical devices rules, 2017 are applicable to substances and devices notified under the drugs and cosmetics act, 1940. Find the latest circulars, notifications and amendments related to the medical devices rules, 2017 issued by the central drugs. A comprehensive overview of the new regulatory framework for medical devices in india, effective from january 1, 2018.
From pharmastate.academy
Medical Devices Regulations 2017, India Explained Medical Devices Rules 2017 A comprehensive overview of the new regulatory framework for medical devices in india, effective from january 1, 2018. This document provides information on the regulatory requirements, quality management systems and standards for medical devices in. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. The medical device rules, 2017. Medical Devices Rules 2017.
From www.flipkart.com
Medical Devices Rules, 2017 As Amended By Medical Devices (Fourth Medical Devices Rules 2017 Find the latest circulars, notifications and amendments related to the medical devices rules, 2017 issued by the central drugs. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. The medical devices rules, 2017 are applicable to substances and devices notified under the drugs and cosmetics act, 1940. A comprehensive. Medical Devices Rules 2017.
From www.youtube.com
New Medical Devices Rules in India 2017 YouTube Medical Devices Rules 2017 Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. A comprehensive overview of the new regulatory framework for medical devices in india, effective from january 1, 2018. The medical devices rules, 2017 are applicable to substances and devices notified under the drugs and cosmetics act, 1940. The medical device. Medical Devices Rules 2017.
From www.slideshare.net
Medical Devices Rules 2017 Implementation Medical Devices Rules 2017 The medical device rules, 2017 asad ullah legislation, sros november 7, 2021 november 7, 2022. A comprehensive overview of the new regulatory framework for medical devices in india, effective from january 1, 2018. The medical devices rules, 2017 are applicable to substances and devices notified under the drugs and cosmetics act, 1940. Regulation (eu) 2017/745 of the european parliament and. Medical Devices Rules 2017.
From www.scribd.com
Medical Devices Rules, 2017 RevisedLatest PDF Medical Devices Rules 2017 The medical device rules, 2017 asad ullah legislation, sros november 7, 2021 november 7, 2022. A comprehensive overview of the new regulatory framework for medical devices in india, effective from january 1, 2018. This document provides information on the regulatory requirements, quality management systems and standards for medical devices in. Regulation (eu) 2017/745 of the european parliament and of the. Medical Devices Rules 2017.
From www.slideserve.com
PPT R egulation of medical devices and diagnostics in India Medical Devices Rules 2017 A comprehensive overview of the new regulatory framework for medical devices in india, effective from january 1, 2018. This document provides information on the regulatory requirements, quality management systems and standards for medical devices in. The medical devices rules, 2017 are applicable to substances and devices notified under the drugs and cosmetics act, 1940. Regulation (eu) 2017/745 of the european. Medical Devices Rules 2017.
From www.slideshare.net
Medical Devices Rules 2017 Implementation Medical Devices Rules 2017 A comprehensive overview of the new regulatory framework for medical devices in india, effective from january 1, 2018. This document provides information on the regulatory requirements, quality management systems and standards for medical devices in. Find the latest circulars, notifications and amendments related to the medical devices rules, 2017 issued by the central drugs. The medical device rules, 2017 asad. Medical Devices Rules 2017.
From cliniexperts.com
Medical Device Grouping as per MDR 2017 CliniExperts CliniExperts Medical Devices Rules 2017 The medical device rules, 2017 asad ullah legislation, sros november 7, 2021 november 7, 2022. A comprehensive overview of the new regulatory framework for medical devices in india, effective from january 1, 2018. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. This document provides information on the regulatory. Medical Devices Rules 2017.
From dcoiwa.com
Drugs Control Officers (I) Welfare Association Medical Devices Rules 2017 A comprehensive overview of the new regulatory framework for medical devices in india, effective from january 1, 2018. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. This document provides information on the regulatory requirements, quality management systems and standards for medical devices in. Find the latest circulars, notifications. Medical Devices Rules 2017.
From www.slideshare.net
Medical Devices Rules 2017 Implementation Medical Devices Rules 2017 The medical device rules, 2017 asad ullah legislation, sros november 7, 2021 november 7, 2022. The medical devices rules, 2017 are applicable to substances and devices notified under the drugs and cosmetics act, 1940. Find the latest circulars, notifications and amendments related to the medical devices rules, 2017 issued by the central drugs. Regulation (eu) 2017/745 of the european parliament. Medical Devices Rules 2017.
From valueadded.in
THE MEDICAL DEVICES RULES, 2017 Value Added Medical Devices Rules 2017 Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. The medical device rules, 2017 asad ullah legislation, sros november 7, 2021 november 7, 2022. A comprehensive overview of the new regulatory framework for medical devices in india, effective from january 1, 2018. The medical devices rules, 2017 are applicable. Medical Devices Rules 2017.
From www.slideserve.com
PPT R egulation of medical devices and diagnostics in India Medical Devices Rules 2017 The medical devices rules, 2017 are applicable to substances and devices notified under the drugs and cosmetics act, 1940. Find the latest circulars, notifications and amendments related to the medical devices rules, 2017 issued by the central drugs. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. A comprehensive. Medical Devices Rules 2017.
From www.slideshare.net
Medical Devices Rules 2017 Implementation Medical Devices Rules 2017 The medical devices rules, 2017 are applicable to substances and devices notified under the drugs and cosmetics act, 1940. The medical device rules, 2017 asad ullah legislation, sros november 7, 2021 november 7, 2022. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. Find the latest circulars, notifications and. Medical Devices Rules 2017.
From www.slideshare.net
Medical Devices Rules 2017 Implementation Medical Devices Rules 2017 This document provides information on the regulatory requirements, quality management systems and standards for medical devices in. The medical devices rules, 2017 are applicable to substances and devices notified under the drugs and cosmetics act, 1940. A comprehensive overview of the new regulatory framework for medical devices in india, effective from january 1, 2018. Find the latest circulars, notifications and. Medical Devices Rules 2017.
From www.scribd.com
Medical Device Rules 2017 Class Download Free PDF Medical Device Medical Devices Rules 2017 The medical device rules, 2017 asad ullah legislation, sros november 7, 2021 november 7, 2022. The medical devices rules, 2017 are applicable to substances and devices notified under the drugs and cosmetics act, 1940. A comprehensive overview of the new regulatory framework for medical devices in india, effective from january 1, 2018. Find the latest circulars, notifications and amendments related. Medical Devices Rules 2017.
From www.slideshare.net
Medical Devices Rules 2017 Implementation Medical Devices Rules 2017 The medical device rules, 2017 asad ullah legislation, sros november 7, 2021 november 7, 2022. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. A comprehensive overview of the new regulatory framework for medical devices in india, effective from january 1, 2018. This document provides information on the regulatory. Medical Devices Rules 2017.
From valueadded.in
Frequently Asked Questions ( FAQs) on Medical Device Rules, 2017 Medical Devices Rules 2017 Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. Find the latest circulars, notifications and amendments related to the medical devices rules, 2017 issued by the central drugs. The medical devices rules, 2017 are applicable to substances and devices notified under the drugs and cosmetics act, 1940. The medical. Medical Devices Rules 2017.
From www.youtube.com
Medical Device Rules, 2017 Implications on medical devices YouTube Medical Devices Rules 2017 A comprehensive overview of the new regulatory framework for medical devices in india, effective from january 1, 2018. The medical device rules, 2017 asad ullah legislation, sros november 7, 2021 november 7, 2022. This document provides information on the regulatory requirements, quality management systems and standards for medical devices in. Find the latest circulars, notifications and amendments related to the. Medical Devices Rules 2017.
From www.slideshare.net
Medical Devices Rules 2017 Implementation Medical Devices Rules 2017 This document provides information on the regulatory requirements, quality management systems and standards for medical devices in. The medical devices rules, 2017 are applicable to substances and devices notified under the drugs and cosmetics act, 1940. The medical device rules, 2017 asad ullah legislation, sros november 7, 2021 november 7, 2022. Find the latest circulars, notifications and amendments related to. Medical Devices Rules 2017.
From gxp-training.com
Medical Device Regulation MDR 2017/745 Course and Certificate Medical Devices Rules 2017 The medical devices rules, 2017 are applicable to substances and devices notified under the drugs and cosmetics act, 1940. The medical device rules, 2017 asad ullah legislation, sros november 7, 2021 november 7, 2022. This document provides information on the regulatory requirements, quality management systems and standards for medical devices in. A comprehensive overview of the new regulatory framework for. Medical Devices Rules 2017.
From www.scribd.com
Medical Device Rules 2017 India PDF Clinical Trial Medical Device Medical Devices Rules 2017 Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. This document provides information on the regulatory requirements, quality management systems and standards for medical devices in. The medical devices rules, 2017 are applicable to substances and devices notified under the drugs and cosmetics act, 1940. The medical device rules,. Medical Devices Rules 2017.
From vircheet.com
Import of Medical Devices as per the requirements of the Medical Medical Devices Rules 2017 A comprehensive overview of the new regulatory framework for medical devices in india, effective from january 1, 2018. The medical devices rules, 2017 are applicable to substances and devices notified under the drugs and cosmetics act, 1940. Find the latest circulars, notifications and amendments related to the medical devices rules, 2017 issued by the central drugs. This document provides information. Medical Devices Rules 2017.
From operonstrategist.com
Medical Devices Rules 2017, the government of Maharashtra Medical Devices Rules 2017 A comprehensive overview of the new regulatory framework for medical devices in india, effective from january 1, 2018. Find the latest circulars, notifications and amendments related to the medical devices rules, 2017 issued by the central drugs. The medical device rules, 2017 asad ullah legislation, sros november 7, 2021 november 7, 2022. This document provides information on the regulatory requirements,. Medical Devices Rules 2017.
From www.scribd.com
Final Medical Devices Rules, 2017 16012018 Final Approved PDF PDF Medical Devices Rules 2017 The medical devices rules, 2017 are applicable to substances and devices notified under the drugs and cosmetics act, 1940. A comprehensive overview of the new regulatory framework for medical devices in india, effective from january 1, 2018. This document provides information on the regulatory requirements, quality management systems and standards for medical devices in. Find the latest circulars, notifications and. Medical Devices Rules 2017.
From www.magzter.com
The Medical Devices Rules, 2017Industry Implications And Action Required Medical Devices Rules 2017 The medical device rules, 2017 asad ullah legislation, sros november 7, 2021 november 7, 2022. A comprehensive overview of the new regulatory framework for medical devices in india, effective from january 1, 2018. The medical devices rules, 2017 are applicable to substances and devices notified under the drugs and cosmetics act, 1940. This document provides information on the regulatory requirements,. Medical Devices Rules 2017.
From www.scconline.com
CDSCO issues classification of Medical Devices pertaining to Urology Medical Devices Rules 2017 A comprehensive overview of the new regulatory framework for medical devices in india, effective from january 1, 2018. Find the latest circulars, notifications and amendments related to the medical devices rules, 2017 issued by the central drugs. This document provides information on the regulatory requirements, quality management systems and standards for medical devices in. Regulation (eu) 2017/745 of the european. Medical Devices Rules 2017.
From www.scribd.com
Medical Device Rules, 2017 PDF Medical Device Verification And Medical Devices Rules 2017 The medical devices rules, 2017 are applicable to substances and devices notified under the drugs and cosmetics act, 1940. The medical device rules, 2017 asad ullah legislation, sros november 7, 2021 november 7, 2022. This document provides information on the regulatory requirements, quality management systems and standards for medical devices in. Find the latest circulars, notifications and amendments related to. Medical Devices Rules 2017.
From www.slideshare.net
Medical Devices Rules 2017 Implementation Medical Devices Rules 2017 Find the latest circulars, notifications and amendments related to the medical devices rules, 2017 issued by the central drugs. A comprehensive overview of the new regulatory framework for medical devices in india, effective from january 1, 2018. The medical devices rules, 2017 are applicable to substances and devices notified under the drugs and cosmetics act, 1940. Regulation (eu) 2017/745 of. Medical Devices Rules 2017.
From www.slideshare.net
Medical Devices Rules 2017 Implementation Medical Devices Rules 2017 Find the latest circulars, notifications and amendments related to the medical devices rules, 2017 issued by the central drugs. A comprehensive overview of the new regulatory framework for medical devices in india, effective from january 1, 2018. The medical devices rules, 2017 are applicable to substances and devices notified under the drugs and cosmetics act, 1940. Regulation (eu) 2017/745 of. Medical Devices Rules 2017.
From www.scribd.com
MedicalDevicesRules2017 PDF Medical Device Health Care Medical Devices Rules 2017 The medical devices rules, 2017 are applicable to substances and devices notified under the drugs and cosmetics act, 1940. A comprehensive overview of the new regulatory framework for medical devices in india, effective from january 1, 2018. The medical device rules, 2017 asad ullah legislation, sros november 7, 2021 november 7, 2022. Find the latest circulars, notifications and amendments related. Medical Devices Rules 2017.
From www.pinterest.com
The Medical Devices Rules, 2017 Industry Implications And Action Medical Devices Rules 2017 The medical device rules, 2017 asad ullah legislation, sros november 7, 2021 november 7, 2022. The medical devices rules, 2017 are applicable to substances and devices notified under the drugs and cosmetics act, 1940. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. Find the latest circulars, notifications and. Medical Devices Rules 2017.
From www.slideshare.net
Medical Devices Rules 2017 Implementation Medical Devices Rules 2017 Find the latest circulars, notifications and amendments related to the medical devices rules, 2017 issued by the central drugs. Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. The medical devices rules, 2017 are applicable to substances and devices notified under the drugs and cosmetics act, 1940. A comprehensive. Medical Devices Rules 2017.
From www.slideshare.net
Medical Devices Rules 2017 Implementation Medical Devices Rules 2017 Regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. A comprehensive overview of the new regulatory framework for medical devices in india, effective from january 1, 2018. The medical devices rules, 2017 are applicable to substances and devices notified under the drugs and cosmetics act, 1940. This document provides. Medical Devices Rules 2017.