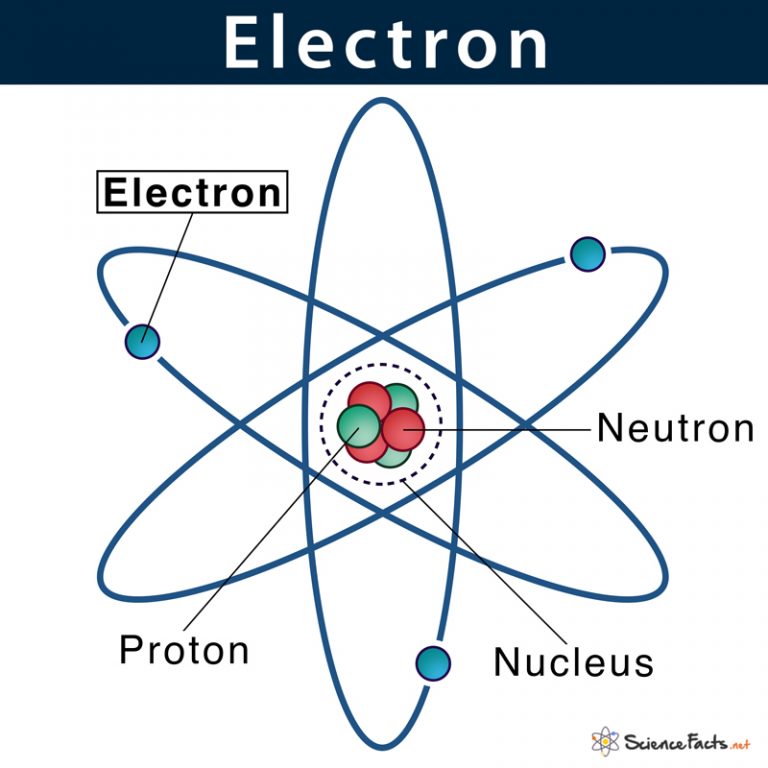
What Repels Electrons From Nucleus . The valence electrons are farther out from the nucleus, so they experience a smaller force of attraction. For this reason, it's usually easier to add neutrons to an atom than to add protons. Although the strong force overcomes electrostatic repulsion, protons do repel each other. Protons and neutrons are in the center of the. This is confirmed by this plot which. Atoms are made of extremely tiny particles called protons, neutrons, and electrons. Shielding refers to the core electrons repelling the outer rings and thus lowering the 1:1. These electrons fill the two available states in the lowest shell,. The force that keeps the electrons near the nucleus is the electrostatic attraction between the electron and the nucleus. Clearly, the electron is more likely to be found the closer we move toward the nucleus.
from www.sciencefacts.net
Although the strong force overcomes electrostatic repulsion, protons do repel each other. Clearly, the electron is more likely to be found the closer we move toward the nucleus. Shielding refers to the core electrons repelling the outer rings and thus lowering the 1:1. The valence electrons are farther out from the nucleus, so they experience a smaller force of attraction. This is confirmed by this plot which. Protons and neutrons are in the center of the. The force that keeps the electrons near the nucleus is the electrostatic attraction between the electron and the nucleus. For this reason, it's usually easier to add neutrons to an atom than to add protons. These electrons fill the two available states in the lowest shell,. Atoms are made of extremely tiny particles called protons, neutrons, and electrons.
Atomic Nucleus Definition, Structure & Parts with Diagram
What Repels Electrons From Nucleus Shielding refers to the core electrons repelling the outer rings and thus lowering the 1:1. Although the strong force overcomes electrostatic repulsion, protons do repel each other. The valence electrons are farther out from the nucleus, so they experience a smaller force of attraction. This is confirmed by this plot which. For this reason, it's usually easier to add neutrons to an atom than to add protons. Clearly, the electron is more likely to be found the closer we move toward the nucleus. These electrons fill the two available states in the lowest shell,. The force that keeps the electrons near the nucleus is the electrostatic attraction between the electron and the nucleus. Protons and neutrons are in the center of the. Shielding refers to the core electrons repelling the outer rings and thus lowering the 1:1. Atoms are made of extremely tiny particles called protons, neutrons, and electrons.
From slideplayer.com
Chapter 7 Periodic Properties of the Elements ppt download What Repels Electrons From Nucleus Clearly, the electron is more likely to be found the closer we move toward the nucleus. Although the strong force overcomes electrostatic repulsion, protons do repel each other. Protons and neutrons are in the center of the. For this reason, it's usually easier to add neutrons to an atom than to add protons. Shielding refers to the core electrons repelling. What Repels Electrons From Nucleus.
From ck12.org
Atomic Forces ( Read ) Physical Science CK12 Foundation What Repels Electrons From Nucleus Protons and neutrons are in the center of the. For this reason, it's usually easier to add neutrons to an atom than to add protons. Atoms are made of extremely tiny particles called protons, neutrons, and electrons. These electrons fill the two available states in the lowest shell,. Shielding refers to the core electrons repelling the outer rings and thus. What Repels Electrons From Nucleus.
From slideplayer.com
What holds an atom together? ppt download What Repels Electrons From Nucleus Atoms are made of extremely tiny particles called protons, neutrons, and electrons. Shielding refers to the core electrons repelling the outer rings and thus lowering the 1:1. Protons and neutrons are in the center of the. The force that keeps the electrons near the nucleus is the electrostatic attraction between the electron and the nucleus. These electrons fill the two. What Repels Electrons From Nucleus.
From www.alamy.com
3d structure of atom showing nucleus , proton, neutron, and electron What Repels Electrons From Nucleus Shielding refers to the core electrons repelling the outer rings and thus lowering the 1:1. Atoms are made of extremely tiny particles called protons, neutrons, and electrons. These electrons fill the two available states in the lowest shell,. The valence electrons are farther out from the nucleus, so they experience a smaller force of attraction. The force that keeps the. What Repels Electrons From Nucleus.
From shaunmwilliams.com
Chapter 2 Presentation What Repels Electrons From Nucleus These electrons fill the two available states in the lowest shell,. Clearly, the electron is more likely to be found the closer we move toward the nucleus. The force that keeps the electrons near the nucleus is the electrostatic attraction between the electron and the nucleus. The valence electrons are farther out from the nucleus, so they experience a smaller. What Repels Electrons From Nucleus.
From stock.adobe.com
Atomic nucleus and the strong force. This science diagram shows the What Repels Electrons From Nucleus These electrons fill the two available states in the lowest shell,. For this reason, it's usually easier to add neutrons to an atom than to add protons. Clearly, the electron is more likely to be found the closer we move toward the nucleus. Protons and neutrons are in the center of the. Shielding refers to the core electrons repelling the. What Repels Electrons From Nucleus.
From www.sciencefacts.net
Atomic Nucleus Definition, Structure & Parts with Diagram What Repels Electrons From Nucleus These electrons fill the two available states in the lowest shell,. For this reason, it's usually easier to add neutrons to an atom than to add protons. The valence electrons are farther out from the nucleus, so they experience a smaller force of attraction. This is confirmed by this plot which. Shielding refers to the core electrons repelling the outer. What Repels Electrons From Nucleus.
From slideplayer.com
CHAPTER 7 Bonding 7.1 What is a Chemical Bond?. ppt download What Repels Electrons From Nucleus The force that keeps the electrons near the nucleus is the electrostatic attraction between the electron and the nucleus. Shielding refers to the core electrons repelling the outer rings and thus lowering the 1:1. Clearly, the electron is more likely to be found the closer we move toward the nucleus. Although the strong force overcomes electrostatic repulsion, protons do repel. What Repels Electrons From Nucleus.
From www.slideserve.com
PPT ATOMIC STRUCTURE PowerPoint Presentation, free download ID4947245 What Repels Electrons From Nucleus Atoms are made of extremely tiny particles called protons, neutrons, and electrons. The force that keeps the electrons near the nucleus is the electrostatic attraction between the electron and the nucleus. Clearly, the electron is more likely to be found the closer we move toward the nucleus. These electrons fill the two available states in the lowest shell,. Shielding refers. What Repels Electrons From Nucleus.
From slideplayer.in.th
Periodic Atomic Properties of the Elements ppt ดาวน์โหลด What Repels Electrons From Nucleus This is confirmed by this plot which. Protons and neutrons are in the center of the. Although the strong force overcomes electrostatic repulsion, protons do repel each other. Clearly, the electron is more likely to be found the closer we move toward the nucleus. For this reason, it's usually easier to add neutrons to an atom than to add protons.. What Repels Electrons From Nucleus.
From saylordotorg.github.io
Lewis Structures and Covalent Bonding What Repels Electrons From Nucleus Although the strong force overcomes electrostatic repulsion, protons do repel each other. Atoms are made of extremely tiny particles called protons, neutrons, and electrons. Clearly, the electron is more likely to be found the closer we move toward the nucleus. The valence electrons are farther out from the nucleus, so they experience a smaller force of attraction. The force that. What Repels Electrons From Nucleus.
From www.slideserve.com
PPT The Periodic Table and Physical Properties PowerPoint What Repels Electrons From Nucleus The valence electrons are farther out from the nucleus, so they experience a smaller force of attraction. Although the strong force overcomes electrostatic repulsion, protons do repel each other. Atoms are made of extremely tiny particles called protons, neutrons, and electrons. Clearly, the electron is more likely to be found the closer we move toward the nucleus. Shielding refers to. What Repels Electrons From Nucleus.
From www.animalia-life.club
Protons What Repels Electrons From Nucleus Although the strong force overcomes electrostatic repulsion, protons do repel each other. Atoms are made of extremely tiny particles called protons, neutrons, and electrons. The valence electrons are farther out from the nucleus, so they experience a smaller force of attraction. This is confirmed by this plot which. Shielding refers to the core electrons repelling the outer rings and thus. What Repels Electrons From Nucleus.
From slideplayer.com
The Structure of the Atom ppt download What Repels Electrons From Nucleus For this reason, it's usually easier to add neutrons to an atom than to add protons. The valence electrons are farther out from the nucleus, so they experience a smaller force of attraction. The force that keeps the electrons near the nucleus is the electrostatic attraction between the electron and the nucleus. This is confirmed by this plot which. Shielding. What Repels Electrons From Nucleus.
From mungfali.com
Periodic Table With Nucleon Number What Repels Electrons From Nucleus These electrons fill the two available states in the lowest shell,. Clearly, the electron is more likely to be found the closer we move toward the nucleus. Although the strong force overcomes electrostatic repulsion, protons do repel each other. For this reason, it's usually easier to add neutrons to an atom than to add protons. Protons and neutrons are in. What Repels Electrons From Nucleus.
From www.youtube.com
Basic Parts of the Atom Protons, Neutrons, Electrons, Nucleus YouTube What Repels Electrons From Nucleus Clearly, the electron is more likely to be found the closer we move toward the nucleus. Shielding refers to the core electrons repelling the outer rings and thus lowering the 1:1. Atoms are made of extremely tiny particles called protons, neutrons, and electrons. The valence electrons are farther out from the nucleus, so they experience a smaller force of attraction.. What Repels Electrons From Nucleus.
From slideplayer.com
The Rutherford Model Known as the “nuclear model”. ppt download What Repels Electrons From Nucleus Although the strong force overcomes electrostatic repulsion, protons do repel each other. These electrons fill the two available states in the lowest shell,. Shielding refers to the core electrons repelling the outer rings and thus lowering the 1:1. The force that keeps the electrons near the nucleus is the electrostatic attraction between the electron and the nucleus. Protons and neutrons. What Repels Electrons From Nucleus.
From slideplayer.com
Atomic Model/Theory Chemistry ppt download What Repels Electrons From Nucleus Atoms are made of extremely tiny particles called protons, neutrons, and electrons. For this reason, it's usually easier to add neutrons to an atom than to add protons. The valence electrons are farther out from the nucleus, so they experience a smaller force of attraction. Clearly, the electron is more likely to be found the closer we move toward the. What Repels Electrons From Nucleus.
From slideplayer.com
Ms. Barlow’s 8th Grade Physical Science Class ppt download What Repels Electrons From Nucleus Protons and neutrons are in the center of the. For this reason, it's usually easier to add neutrons to an atom than to add protons. Although the strong force overcomes electrostatic repulsion, protons do repel each other. The valence electrons are farther out from the nucleus, so they experience a smaller force of attraction. The force that keeps the electrons. What Repels Electrons From Nucleus.
From www.pinterest.com
Strong force in the nucleus of an atom . This science diagram shows the What Repels Electrons From Nucleus Atoms are made of extremely tiny particles called protons, neutrons, and electrons. Protons and neutrons are in the center of the. The valence electrons are farther out from the nucleus, so they experience a smaller force of attraction. This is confirmed by this plot which. These electrons fill the two available states in the lowest shell,. Clearly, the electron is. What Repels Electrons From Nucleus.
From electronicsgurukulam.blogspot.com
ELECTRONICS GURUKULAM Basics of Electronics What Repels Electrons From Nucleus These electrons fill the two available states in the lowest shell,. Atoms are made of extremely tiny particles called protons, neutrons, and electrons. Shielding refers to the core electrons repelling the outer rings and thus lowering the 1:1. The valence electrons are farther out from the nucleus, so they experience a smaller force of attraction. Protons and neutrons are in. What Repels Electrons From Nucleus.
From www.alamy.com
Atomic structural electrons orbits nucleus proton neutron electrons What Repels Electrons From Nucleus Protons and neutrons are in the center of the. The force that keeps the electrons near the nucleus is the electrostatic attraction between the electron and the nucleus. Although the strong force overcomes electrostatic repulsion, protons do repel each other. This is confirmed by this plot which. Atoms are made of extremely tiny particles called protons, neutrons, and electrons. Shielding. What Repels Electrons From Nucleus.
From slideplayer.com
Electronic Structure of the Atom ppt download What Repels Electrons From Nucleus Clearly, the electron is more likely to be found the closer we move toward the nucleus. These electrons fill the two available states in the lowest shell,. This is confirmed by this plot which. Shielding refers to the core electrons repelling the outer rings and thus lowering the 1:1. The force that keeps the electrons near the nucleus is the. What Repels Electrons From Nucleus.
From www.chemistrystudent.com
Electron Orbitals (ALevel) ChemistryStudent What Repels Electrons From Nucleus For this reason, it's usually easier to add neutrons to an atom than to add protons. Protons and neutrons are in the center of the. The valence electrons are farther out from the nucleus, so they experience a smaller force of attraction. Although the strong force overcomes electrostatic repulsion, protons do repel each other. Clearly, the electron is more likely. What Repels Electrons From Nucleus.
From www.youtube.com
Electrons revolving around nucleus (Animation)HD 1080p[short video What Repels Electrons From Nucleus This is confirmed by this plot which. For this reason, it's usually easier to add neutrons to an atom than to add protons. The valence electrons are farther out from the nucleus, so they experience a smaller force of attraction. Although the strong force overcomes electrostatic repulsion, protons do repel each other. Protons and neutrons are in the center of. What Repels Electrons From Nucleus.
From www.slideserve.com
PPT ATOMS Dalton and Beyond A search for a simple theory of matter What Repels Electrons From Nucleus The valence electrons are farther out from the nucleus, so they experience a smaller force of attraction. Clearly, the electron is more likely to be found the closer we move toward the nucleus. Protons and neutrons are in the center of the. Although the strong force overcomes electrostatic repulsion, protons do repel each other. Shielding refers to the core electrons. What Repels Electrons From Nucleus.
From www.shutterstock.com
Simple Model Atom Structure Electrons Orbiting Stock Vector (Royalty What Repels Electrons From Nucleus Atoms are made of extremely tiny particles called protons, neutrons, and electrons. The valence electrons are farther out from the nucleus, so they experience a smaller force of attraction. These electrons fill the two available states in the lowest shell,. This is confirmed by this plot which. Protons and neutrons are in the center of the. Although the strong force. What Repels Electrons From Nucleus.
From slideplayer.com
Electrostatics Fundamental Forces Model of the atom Unit of charge What Repels Electrons From Nucleus The valence electrons are farther out from the nucleus, so they experience a smaller force of attraction. For this reason, it's usually easier to add neutrons to an atom than to add protons. Although the strong force overcomes electrostatic repulsion, protons do repel each other. Atoms are made of extremely tiny particles called protons, neutrons, and electrons. Clearly, the electron. What Repels Electrons From Nucleus.
From www.pathwaystochemistry.com
Atomic Structure Pathways to Chemistry What Repels Electrons From Nucleus Shielding refers to the core electrons repelling the outer rings and thus lowering the 1:1. Clearly, the electron is more likely to be found the closer we move toward the nucleus. This is confirmed by this plot which. The valence electrons are farther out from the nucleus, so they experience a smaller force of attraction. These electrons fill the two. What Repels Electrons From Nucleus.
From www.cyberphysics.co.uk
The nucleus What Repels Electrons From Nucleus Clearly, the electron is more likely to be found the closer we move toward the nucleus. This is confirmed by this plot which. These electrons fill the two available states in the lowest shell,. Although the strong force overcomes electrostatic repulsion, protons do repel each other. For this reason, it's usually easier to add neutrons to an atom than to. What Repels Electrons From Nucleus.
From stock.adobe.com
illustration of physics and chemistry, Coulomb's law, protons repel What Repels Electrons From Nucleus Protons and neutrons are in the center of the. The force that keeps the electrons near the nucleus is the electrostatic attraction between the electron and the nucleus. Atoms are made of extremely tiny particles called protons, neutrons, and electrons. This is confirmed by this plot which. Clearly, the electron is more likely to be found the closer we move. What Repels Electrons From Nucleus.
From www.youtube.com
Arrangement of Particles in the Atom Nucleus of Atom Atomic What Repels Electrons From Nucleus These electrons fill the two available states in the lowest shell,. This is confirmed by this plot which. The force that keeps the electrons near the nucleus is the electrostatic attraction between the electron and the nucleus. For this reason, it's usually easier to add neutrons to an atom than to add protons. Protons and neutrons are in the center. What Repels Electrons From Nucleus.
From science-education-research.com
Electrons repel each other, keeping them out of the nucleus Science What Repels Electrons From Nucleus Clearly, the electron is more likely to be found the closer we move toward the nucleus. These electrons fill the two available states in the lowest shell,. The valence electrons are farther out from the nucleus, so they experience a smaller force of attraction. Shielding refers to the core electrons repelling the outer rings and thus lowering the 1:1. The. What Repels Electrons From Nucleus.
From spmscience.blog.onlinetuition.com.my
(A) The composition of the Nucleus SPM Science What Repels Electrons From Nucleus Although the strong force overcomes electrostatic repulsion, protons do repel each other. The force that keeps the electrons near the nucleus is the electrostatic attraction between the electron and the nucleus. Clearly, the electron is more likely to be found the closer we move toward the nucleus. Shielding refers to the core electrons repelling the outer rings and thus lowering. What Repels Electrons From Nucleus.
From www.broadlearnings.com
Atomic Structure Broad Learnings What Repels Electrons From Nucleus Shielding refers to the core electrons repelling the outer rings and thus lowering the 1:1. This is confirmed by this plot which. For this reason, it's usually easier to add neutrons to an atom than to add protons. The force that keeps the electrons near the nucleus is the electrostatic attraction between the electron and the nucleus. Clearly, the electron. What Repels Electrons From Nucleus.