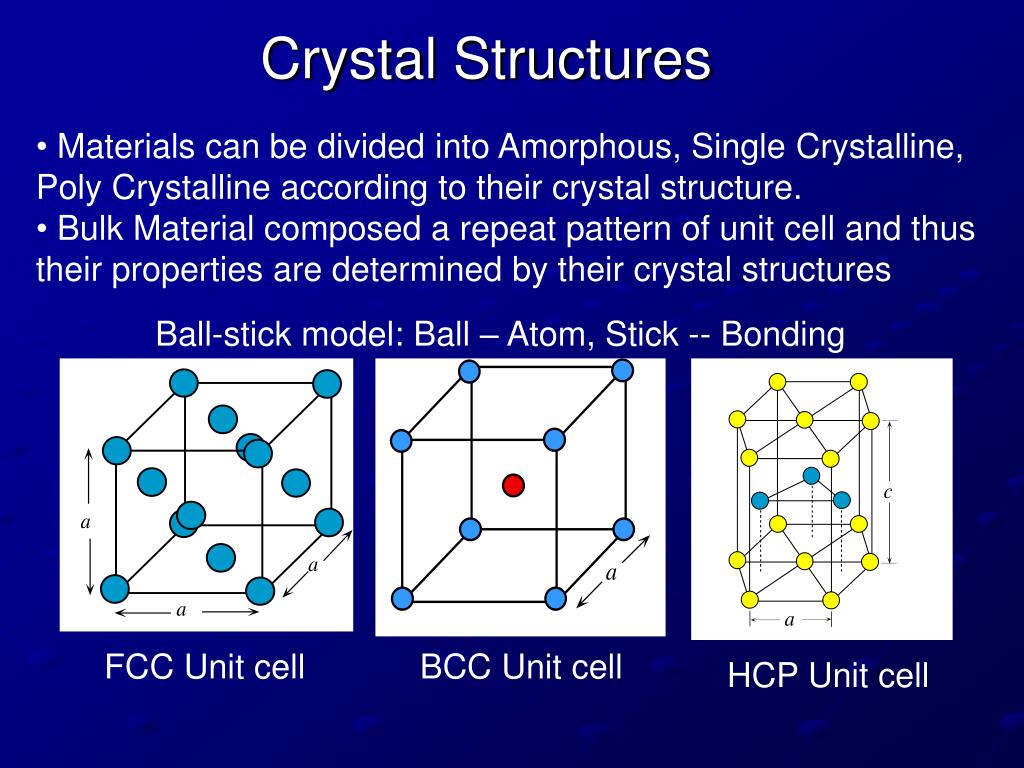
Define Crystal Class 7 . Crystallization occurs in two major steps. The process of obtaining crystals of pure substances from their solutions is called crystallisation. Crystallization is a process by which a pure soluble substance separates out in the form of crystals from its hot and saturated solution on cooling. The process is employed in the. The interaction between individual crystals can influence the final morphology and size distribution of the crystal population. Every point in a crystal structure is defined as a lattice point or lattice site. Understanding the aggregation process is. Crystallization is a natural process which happens when the materials solidify from a liquid, or as they precipitate out of a liquid or gas. The first one is nucleation, which gives the looks of a crystalline phase from either a supercooled liquid or a supersaturated solvent. It is the process used to obtain. Two or more lattice points can be joined to form a straight line.
from exokbmzkz.blob.core.windows.net
The first one is nucleation, which gives the looks of a crystalline phase from either a supercooled liquid or a supersaturated solvent. The process is employed in the. Two or more lattice points can be joined to form a straight line. It is the process used to obtain. Crystallization occurs in two major steps. Crystallization is a natural process which happens when the materials solidify from a liquid, or as they precipitate out of a liquid or gas. The interaction between individual crystals can influence the final morphology and size distribution of the crystal population. Understanding the aggregation process is. Every point in a crystal structure is defined as a lattice point or lattice site. Crystallization is a process by which a pure soluble substance separates out in the form of crystals from its hot and saturated solution on cooling.
Crystalline Atoms Definition at Priscilla Anderson blog
Define Crystal Class 7 The interaction between individual crystals can influence the final morphology and size distribution of the crystal population. Crystallization occurs in two major steps. The first one is nucleation, which gives the looks of a crystalline phase from either a supercooled liquid or a supersaturated solvent. Crystallization is a process by which a pure soluble substance separates out in the form of crystals from its hot and saturated solution on cooling. Two or more lattice points can be joined to form a straight line. The interaction between individual crystals can influence the final morphology and size distribution of the crystal population. It is the process used to obtain. Crystallization is a natural process which happens when the materials solidify from a liquid, or as they precipitate out of a liquid or gas. The process of obtaining crystals of pure substances from their solutions is called crystallisation. Understanding the aggregation process is. The process is employed in the. Every point in a crystal structure is defined as a lattice point or lattice site.
From www.youtube.com
Trick to remember 7 crystal systems / Solid State/ Class 12 Chemistry Define Crystal Class 7 Crystallization is a natural process which happens when the materials solidify from a liquid, or as they precipitate out of a liquid or gas. Understanding the aggregation process is. Crystallization occurs in two major steps. The process is employed in the. Two or more lattice points can be joined to form a straight line. Every point in a crystal structure. Define Crystal Class 7.
From www.youtube.com
Unit 3.6 Crystal Classes (II) YouTube Define Crystal Class 7 The process of obtaining crystals of pure substances from their solutions is called crystallisation. Crystallization occurs in two major steps. The first one is nucleation, which gives the looks of a crystalline phase from either a supercooled liquid or a supersaturated solvent. The process is employed in the. The interaction between individual crystals can influence the final morphology and size. Define Crystal Class 7.
From www.youtube.com
Classification of Crystals into Classes “Elements of Symmetry” YouTube Define Crystal Class 7 Understanding the aggregation process is. Crystallization occurs in two major steps. Every point in a crystal structure is defined as a lattice point or lattice site. The interaction between individual crystals can influence the final morphology and size distribution of the crystal population. Crystallization is a natural process which happens when the materials solidify from a liquid, or as they. Define Crystal Class 7.
From roywmacdonald.com
RevisedSevenCrystalSystems Diamond Define Crystal Class 7 The process of obtaining crystals of pure substances from their solutions is called crystallisation. Every point in a crystal structure is defined as a lattice point or lattice site. Crystallization is a natural process which happens when the materials solidify from a liquid, or as they precipitate out of a liquid or gas. Crystallization occurs in two major steps. The. Define Crystal Class 7.
From www.findlight.net
XRay Diffraction Getting to Know Crystal Structures (Part Ⅰ) Define Crystal Class 7 The first one is nucleation, which gives the looks of a crystalline phase from either a supercooled liquid or a supersaturated solvent. Two or more lattice points can be joined to form a straight line. The process is employed in the. The process of obtaining crystals of pure substances from their solutions is called crystallisation. Crystallization is a process by. Define Crystal Class 7.
From www.youtube.com
4types of Crystals CBSE Class XII UNIT1 chemistry cbse YouTube Define Crystal Class 7 Crystallization occurs in two major steps. Two or more lattice points can be joined to form a straight line. It is the process used to obtain. The process of obtaining crystals of pure substances from their solutions is called crystallisation. Understanding the aggregation process is. Every point in a crystal structure is defined as a lattice point or lattice site.. Define Crystal Class 7.
From chemistryskills.com
Classification of crystals Chemistry Skills Define Crystal Class 7 Crystallization is a natural process which happens when the materials solidify from a liquid, or as they precipitate out of a liquid or gas. Every point in a crystal structure is defined as a lattice point or lattice site. It is the process used to obtain. Crystallization occurs in two major steps. Two or more lattice points can be joined. Define Crystal Class 7.
From www.researchgate.net
Classification of crystal class Download Scientific Diagram Define Crystal Class 7 Two or more lattice points can be joined to form a straight line. It is the process used to obtain. Crystallization is a natural process which happens when the materials solidify from a liquid, or as they precipitate out of a liquid or gas. Crystallization is a process by which a pure soluble substance separates out in the form of. Define Crystal Class 7.
From www.britannica.com
isometric system Definition & Facts Britannica Define Crystal Class 7 It is the process used to obtain. Crystallization is a process by which a pure soluble substance separates out in the form of crystals from its hot and saturated solution on cooling. Crystallization is a natural process which happens when the materials solidify from a liquid, or as they precipitate out of a liquid or gas. The process of obtaining. Define Crystal Class 7.
From ar.inspiredpencil.com
Trigonal Crystal System Define Crystal Class 7 Two or more lattice points can be joined to form a straight line. Crystallization is a natural process which happens when the materials solidify from a liquid, or as they precipitate out of a liquid or gas. The interaction between individual crystals can influence the final morphology and size distribution of the crystal population. Every point in a crystal structure. Define Crystal Class 7.
From www.pinterest.com
Crystal Classes Define Crystal Class 7 It is the process used to obtain. The first one is nucleation, which gives the looks of a crystalline phase from either a supercooled liquid or a supersaturated solvent. The process of obtaining crystals of pure substances from their solutions is called crystallisation. Two or more lattice points can be joined to form a straight line. Every point in a. Define Crystal Class 7.
From www.luckysci.com
Best trick for learning the seven crystal systems Lucky Sci Define Crystal Class 7 Crystallization is a process by which a pure soluble substance separates out in the form of crystals from its hot and saturated solution on cooling. Two or more lattice points can be joined to form a straight line. It is the process used to obtain. Every point in a crystal structure is defined as a lattice point or lattice site.. Define Crystal Class 7.
From www.youtube.com
Unit 1.8 The Seven Crystal Systems YouTube Define Crystal Class 7 Crystallization is a process by which a pure soluble substance separates out in the form of crystals from its hot and saturated solution on cooling. The first one is nucleation, which gives the looks of a crystalline phase from either a supercooled liquid or a supersaturated solvent. Crystallization occurs in two major steps. Crystallization is a natural process which happens. Define Crystal Class 7.
From www.slideserve.com
PPT Lecture 3 Rocks and Minerals PowerPoint Presentation, free Define Crystal Class 7 The process of obtaining crystals of pure substances from their solutions is called crystallisation. Crystallization is a process by which a pure soluble substance separates out in the form of crystals from its hot and saturated solution on cooling. Crystallization occurs in two major steps. Understanding the aggregation process is. The interaction between individual crystals can influence the final morphology. Define Crystal Class 7.
From www.facebook.com
Wednesday Devo & Class 10/16/2024 to the Wednesday night Define Crystal Class 7 It is the process used to obtain. Crystallization is a process by which a pure soluble substance separates out in the form of crystals from its hot and saturated solution on cooling. The interaction between individual crystals can influence the final morphology and size distribution of the crystal population. Crystallization occurs in two major steps. The first one is nucleation,. Define Crystal Class 7.
From www.youtube.com
What are different types of crystals. Solid State Physical Define Crystal Class 7 It is the process used to obtain. Crystallization is a natural process which happens when the materials solidify from a liquid, or as they precipitate out of a liquid or gas. Crystallization occurs in two major steps. Every point in a crystal structure is defined as a lattice point or lattice site. The process is employed in the. The process. Define Crystal Class 7.
From ancient-tech.com
7 Crystal Systems Class Ancient Technologies Define Crystal Class 7 The process is employed in the. Crystallization occurs in two major steps. Every point in a crystal structure is defined as a lattice point or lattice site. The first one is nucleation, which gives the looks of a crystalline phase from either a supercooled liquid or a supersaturated solvent. Crystallization is a natural process which happens when the materials solidify. Define Crystal Class 7.
From msestudent.com
The 7 Crystal Systems (with Examples and Images) Materials Science Define Crystal Class 7 Crystallization is a process by which a pure soluble substance separates out in the form of crystals from its hot and saturated solution on cooling. Every point in a crystal structure is defined as a lattice point or lattice site. Crystallization is a natural process which happens when the materials solidify from a liquid, or as they precipitate out of. Define Crystal Class 7.
From www.geologyin.com
Crystal Habits and Forms Geology In Define Crystal Class 7 Crystallization is a process by which a pure soluble substance separates out in the form of crystals from its hot and saturated solution on cooling. It is the process used to obtain. Understanding the aggregation process is. Crystallization is a natural process which happens when the materials solidify from a liquid, or as they precipitate out of a liquid or. Define Crystal Class 7.
From www.slideserve.com
PPT Guided Notes about Mineral Formation PowerPoint Presentation Define Crystal Class 7 Understanding the aggregation process is. It is the process used to obtain. The first one is nucleation, which gives the looks of a crystalline phase from either a supercooled liquid or a supersaturated solvent. Every point in a crystal structure is defined as a lattice point or lattice site. The interaction between individual crystals can influence the final morphology and. Define Crystal Class 7.
From brainly.com
Which correctly lists two characteristics of crystal faces that define Define Crystal Class 7 Understanding the aggregation process is. The interaction between individual crystals can influence the final morphology and size distribution of the crystal population. It is the process used to obtain. Two or more lattice points can be joined to form a straight line. The process is employed in the. The first one is nucleation, which gives the looks of a crystalline. Define Crystal Class 7.
From study.com
Crystal Structures and the Unit Cell Lesson Define Crystal Class 7 Crystallization is a natural process which happens when the materials solidify from a liquid, or as they precipitate out of a liquid or gas. Every point in a crystal structure is defined as a lattice point or lattice site. The interaction between individual crystals can influence the final morphology and size distribution of the crystal population. Two or more lattice. Define Crystal Class 7.
From www.youtube.com
Trick to Remember 7 crystal systems Solid State Class 12 Chemistry Define Crystal Class 7 Crystallization occurs in two major steps. Two or more lattice points can be joined to form a straight line. Crystallization is a process by which a pure soluble substance separates out in the form of crystals from its hot and saturated solution on cooling. The first one is nucleation, which gives the looks of a crystalline phase from either a. Define Crystal Class 7.
From chemistryskills.com
Classification of crystals Chemistry Skills Define Crystal Class 7 Crystallization is a natural process which happens when the materials solidify from a liquid, or as they precipitate out of a liquid or gas. The process is employed in the. Crystallization is a process by which a pure soluble substance separates out in the form of crystals from its hot and saturated solution on cooling. Understanding the aggregation process is.. Define Crystal Class 7.
From exokbmzkz.blob.core.windows.net
Crystalline Atoms Definition at Priscilla Anderson blog Define Crystal Class 7 Crystallization is a natural process which happens when the materials solidify from a liquid, or as they precipitate out of a liquid or gas. The first one is nucleation, which gives the looks of a crystalline phase from either a supercooled liquid or a supersaturated solvent. The interaction between individual crystals can influence the final morphology and size distribution of. Define Crystal Class 7.
From www.britannica.com
Mineral Crystal Habit, Aggregation Britannica Define Crystal Class 7 The first one is nucleation, which gives the looks of a crystalline phase from either a supercooled liquid or a supersaturated solvent. Two or more lattice points can be joined to form a straight line. Every point in a crystal structure is defined as a lattice point or lattice site. The process is employed in the. Crystallization is a natural. Define Crystal Class 7.
From www.slideserve.com
PPT Xray Diffraction (XRD) PowerPoint Presentation ID242858 Define Crystal Class 7 Crystallization is a natural process which happens when the materials solidify from a liquid, or as they precipitate out of a liquid or gas. The interaction between individual crystals can influence the final morphology and size distribution of the crystal population. Understanding the aggregation process is. Crystallization is a process by which a pure soluble substance separates out in the. Define Crystal Class 7.
From www.howlatm.com
Intro to Crystals Howl at the Moon Gems Define Crystal Class 7 Understanding the aggregation process is. The first one is nucleation, which gives the looks of a crystalline phase from either a supercooled liquid or a supersaturated solvent. Crystallization occurs in two major steps. It is the process used to obtain. The process of obtaining crystals of pure substances from their solutions is called crystallisation. Two or more lattice points can. Define Crystal Class 7.
From study.com
Crystal Definition, Types & Uses Lesson Define Crystal Class 7 The first one is nucleation, which gives the looks of a crystalline phase from either a supercooled liquid or a supersaturated solvent. The interaction between individual crystals can influence the final morphology and size distribution of the crystal population. Crystallization is a process by which a pure soluble substance separates out in the form of crystals from its hot and. Define Crystal Class 7.
From www.britannica.com
Symmetry Crystal Classes & Facts Britannica Define Crystal Class 7 Every point in a crystal structure is defined as a lattice point or lattice site. Crystallization occurs in two major steps. The first one is nucleation, which gives the looks of a crystalline phase from either a supercooled liquid or a supersaturated solvent. Crystallization is a process by which a pure soluble substance separates out in the form of crystals. Define Crystal Class 7.
From home.iitk.ac.in
Basic Crystal Concepts Define Crystal Class 7 Understanding the aggregation process is. The process is employed in the. The first one is nucleation, which gives the looks of a crystalline phase from either a supercooled liquid or a supersaturated solvent. Crystallization occurs in two major steps. Two or more lattice points can be joined to form a straight line. Crystallization is a process by which a pure. Define Crystal Class 7.
From www.geologyin.com
Crystal Formations and Their Meanings Geology In Define Crystal Class 7 The interaction between individual crystals can influence the final morphology and size distribution of the crystal population. The process is employed in the. Two or more lattice points can be joined to form a straight line. Understanding the aggregation process is. Every point in a crystal structure is defined as a lattice point or lattice site. The process of obtaining. Define Crystal Class 7.
From www.geologyin.com
Crystal Systems and Crystal Structure Geology In Define Crystal Class 7 The process is employed in the. It is the process used to obtain. The process of obtaining crystals of pure substances from their solutions is called crystallisation. The interaction between individual crystals can influence the final morphology and size distribution of the crystal population. Crystallization is a natural process which happens when the materials solidify from a liquid, or as. Define Crystal Class 7.
From www.geologyin.com
Crystal Structure and Crystal Systems Define Crystal Class 7 It is the process used to obtain. The process is employed in the. Every point in a crystal structure is defined as a lattice point or lattice site. Crystallization is a natural process which happens when the materials solidify from a liquid, or as they precipitate out of a liquid or gas. The first one is nucleation, which gives the. Define Crystal Class 7.
From www.pinterest.com
The Seven Crystal Systems A Guide to Axial Lengths and Angles Define Crystal Class 7 Understanding the aggregation process is. Two or more lattice points can be joined to form a straight line. The process is employed in the. Crystallization occurs in two major steps. The first one is nucleation, which gives the looks of a crystalline phase from either a supercooled liquid or a supersaturated solvent. Crystallization is a process by which a pure. Define Crystal Class 7.