Pi Bonds Equation . The pi bond (π bond) has two halves—one above the plane of the molecule, and the other below it. Are pi bonds stronger than sigma bonds? Notice how the orientation of. Pi bonds \((\pi)\) are a type of covalent bond formed by sideways or lateral overlapping of atomic orbitals. Illustration of a pi bond forming from two p orbitals. Each of the two electrons in the pi bond (π bond) exists both above and below the plane of. Pi bonds are chemical bonds that are covalent in nature and involve the lateral overlapping of two lobes of an atomic orbital with two lobes of another atomic orbital that belongs to a. What is the difference between a sigma bond and a pi bond?
from www.vedantu.com
Notice how the orientation of. Are pi bonds stronger than sigma bonds? Pi bonds are chemical bonds that are covalent in nature and involve the lateral overlapping of two lobes of an atomic orbital with two lobes of another atomic orbital that belongs to a. Pi bonds \((\pi)\) are a type of covalent bond formed by sideways or lateral overlapping of atomic orbitals. Illustration of a pi bond forming from two p orbitals. The pi bond (π bond) has two halves—one above the plane of the molecule, and the other below it. What is the difference between a sigma bond and a pi bond? Each of the two electrons in the pi bond (π bond) exists both above and below the plane of.
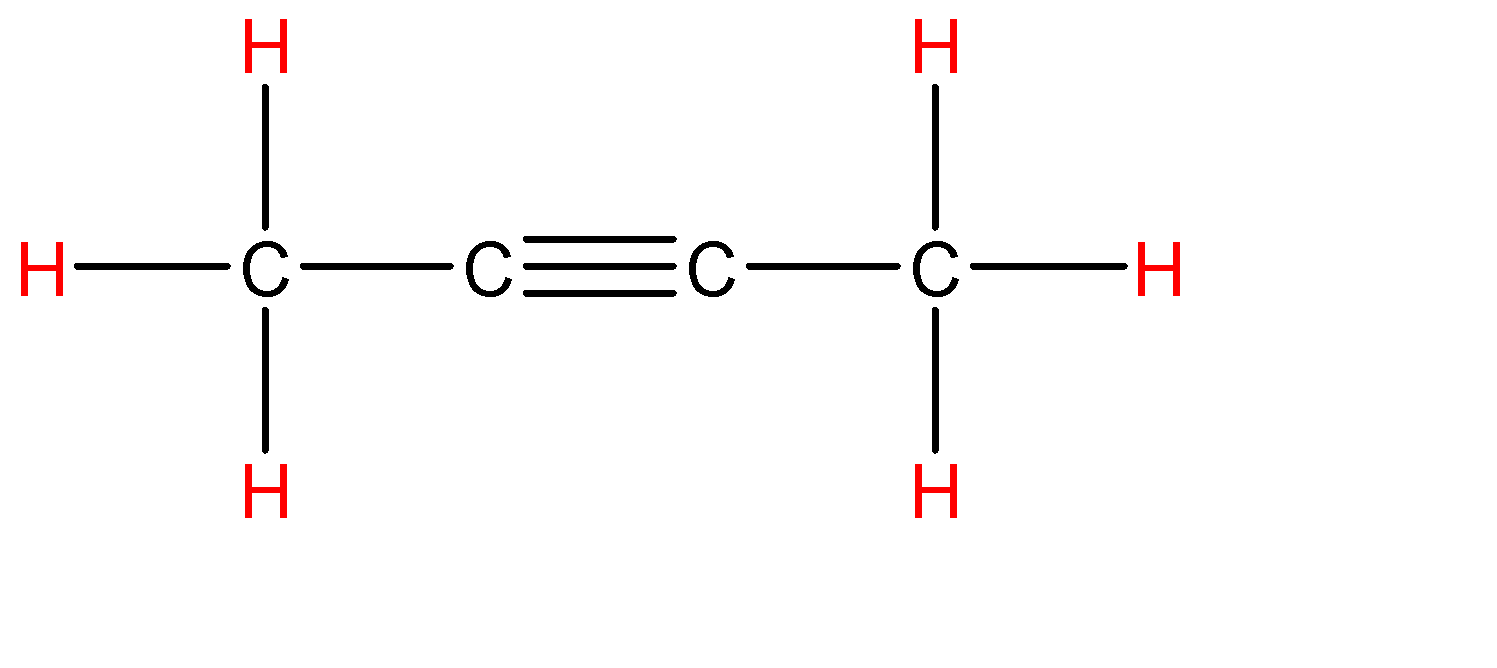
How many sigma and pi bonds are in {{C}_{4}}{{H}_{6}}
Pi Bonds Equation The pi bond (π bond) has two halves—one above the plane of the molecule, and the other below it. Are pi bonds stronger than sigma bonds? Pi bonds are chemical bonds that are covalent in nature and involve the lateral overlapping of two lobes of an atomic orbital with two lobes of another atomic orbital that belongs to a. Pi bonds \((\pi)\) are a type of covalent bond formed by sideways or lateral overlapping of atomic orbitals. Each of the two electrons in the pi bond (π bond) exists both above and below the plane of. Notice how the orientation of. What is the difference between a sigma bond and a pi bond? The pi bond (π bond) has two halves—one above the plane of the molecule, and the other below it. Illustration of a pi bond forming from two p orbitals.
From ar.inspiredpencil.com
Sigma And Pi Bonds Explained Pi Bonds Equation Pi bonds \((\pi)\) are a type of covalent bond formed by sideways or lateral overlapping of atomic orbitals. Illustration of a pi bond forming from two p orbitals. Notice how the orientation of. Pi bonds are chemical bonds that are covalent in nature and involve the lateral overlapping of two lobes of an atomic orbital with two lobes of another. Pi Bonds Equation.
From www.youtube.com
JSSC CGL !!SIGMA BOND ,PIE BOND, CHEMICAL FORMULA σ bonds और π bond Pi Bonds Equation Illustration of a pi bond forming from two p orbitals. Each of the two electrons in the pi bond (π bond) exists both above and below the plane of. Are pi bonds stronger than sigma bonds? Pi bonds \((\pi)\) are a type of covalent bond formed by sideways or lateral overlapping of atomic orbitals. Notice how the orientation of. Pi. Pi Bonds Equation.
From brainly.in
How many sigma bond and pi bonds are present In CH2=C=CH CH3 Brainly.in Pi Bonds Equation Pi bonds are chemical bonds that are covalent in nature and involve the lateral overlapping of two lobes of an atomic orbital with two lobes of another atomic orbital that belongs to a. What is the difference between a sigma bond and a pi bond? The pi bond (π bond) has two halves—one above the plane of the molecule, and. Pi Bonds Equation.
From slideplayer.com
Energy, Bonds & Chemical Structure ppt download Pi Bonds Equation What is the difference between a sigma bond and a pi bond? Pi bonds \((\pi)\) are a type of covalent bond formed by sideways or lateral overlapping of atomic orbitals. Notice how the orientation of. The pi bond (π bond) has two halves—one above the plane of the molecule, and the other below it. Pi bonds are chemical bonds that. Pi Bonds Equation.
From www.chemistrysteps.com
Alkenes Structure and Stability Chemistry Steps Pi Bonds Equation Pi bonds \((\pi)\) are a type of covalent bond formed by sideways or lateral overlapping of atomic orbitals. Are pi bonds stronger than sigma bonds? Each of the two electrons in the pi bond (π bond) exists both above and below the plane of. What is the difference between a sigma bond and a pi bond? Pi bonds are chemical. Pi Bonds Equation.
From brainly.com
Give the number of sigma bonds and pi bonds for benzene, c6h6 Pi Bonds Equation Illustration of a pi bond forming from two p orbitals. Pi bonds \((\pi)\) are a type of covalent bond formed by sideways or lateral overlapping of atomic orbitals. Notice how the orientation of. Are pi bonds stronger than sigma bonds? What is the difference between a sigma bond and a pi bond? Pi bonds are chemical bonds that are covalent. Pi Bonds Equation.
From slideplayer.com
Chapter 7 Ionic & Covalent Bonds. ppt download Pi Bonds Equation What is the difference between a sigma bond and a pi bond? Each of the two electrons in the pi bond (π bond) exists both above and below the plane of. Notice how the orientation of. Are pi bonds stronger than sigma bonds? Pi bonds are chemical bonds that are covalent in nature and involve the lateral overlapping of two. Pi Bonds Equation.
From www.numerade.com
SOLVED How many rings and π(pi) bonds are contained in compound A and Pi Bonds Equation Each of the two electrons in the pi bond (π bond) exists both above and below the plane of. Pi bonds are chemical bonds that are covalent in nature and involve the lateral overlapping of two lobes of an atomic orbital with two lobes of another atomic orbital that belongs to a. What is the difference between a sigma bond. Pi Bonds Equation.
From socratic.org
How many sigma bonds are in benzene? Socratic Pi Bonds Equation The pi bond (π bond) has two halves—one above the plane of the molecule, and the other below it. Illustration of a pi bond forming from two p orbitals. Notice how the orientation of. Pi bonds \((\pi)\) are a type of covalent bond formed by sideways or lateral overlapping of atomic orbitals. Pi bonds are chemical bonds that are covalent. Pi Bonds Equation.
From byjus.com
Which of the following species contains equal number of σ and π bonds? Pi Bonds Equation Pi bonds \((\pi)\) are a type of covalent bond formed by sideways or lateral overlapping of atomic orbitals. Notice how the orientation of. The pi bond (π bond) has two halves—one above the plane of the molecule, and the other below it. Each of the two electrons in the pi bond (π bond) exists both above and below the plane. Pi Bonds Equation.
From www.chegg.com
Solved Determine the relationship between the two compounds Pi Bonds Equation Pi bonds are chemical bonds that are covalent in nature and involve the lateral overlapping of two lobes of an atomic orbital with two lobes of another atomic orbital that belongs to a. What is the difference between a sigma bond and a pi bond? Each of the two electrons in the pi bond (π bond) exists both above and. Pi Bonds Equation.
From www.youtube.com
Trick To Find Number Of Sigma and Pi Bonds YouTube Pi Bonds Equation Each of the two electrons in the pi bond (π bond) exists both above and below the plane of. Illustration of a pi bond forming from two p orbitals. Are pi bonds stronger than sigma bonds? What is the difference between a sigma bond and a pi bond? Pi bonds are chemical bonds that are covalent in nature and involve. Pi Bonds Equation.
From www.coursehero.com
[Solved] 1. How many sigma and pi bonds? 2. Molecular Formula?. 4 / HO Pi Bonds Equation Each of the two electrons in the pi bond (π bond) exists both above and below the plane of. Pi bonds \((\pi)\) are a type of covalent bond formed by sideways or lateral overlapping of atomic orbitals. Illustration of a pi bond forming from two p orbitals. Are pi bonds stronger than sigma bonds? Pi bonds are chemical bonds that. Pi Bonds Equation.
From www.numerade.com
SOLVEDSigma bonds and pi bonds are classifications of covalent bonds Pi Bonds Equation Notice how the orientation of. Pi bonds \((\pi)\) are a type of covalent bond formed by sideways or lateral overlapping of atomic orbitals. Are pi bonds stronger than sigma bonds? What is the difference between a sigma bond and a pi bond? Illustration of a pi bond forming from two p orbitals. The pi bond (π bond) has two halves—one. Pi Bonds Equation.
From www.chegg.com
Solved How do you derive/solve the E_bond equation in bottom Pi Bonds Equation Pi bonds \((\pi)\) are a type of covalent bond formed by sideways or lateral overlapping of atomic orbitals. Each of the two electrons in the pi bond (π bond) exists both above and below the plane of. What is the difference between a sigma bond and a pi bond? Illustration of a pi bond forming from two p orbitals. The. Pi Bonds Equation.
From www.chegg.com
Solved Determine the net number of sigma bonds, the net Pi Bonds Equation What is the difference between a sigma bond and a pi bond? Are pi bonds stronger than sigma bonds? The pi bond (π bond) has two halves—one above the plane of the molecule, and the other below it. Illustration of a pi bond forming from two p orbitals. Pi bonds \((\pi)\) are a type of covalent bond formed by sideways. Pi Bonds Equation.
From slideplayer.com
Covalent Bonding. ppt download Pi Bonds Equation Illustration of a pi bond forming from two p orbitals. The pi bond (π bond) has two halves—one above the plane of the molecule, and the other below it. Pi bonds \((\pi)\) are a type of covalent bond formed by sideways or lateral overlapping of atomic orbitals. What is the difference between a sigma bond and a pi bond? Notice. Pi Bonds Equation.
From www.youtube.com
How to find sigma and pi bond in easy way ! YouTube Pi Bonds Equation Are pi bonds stronger than sigma bonds? Pi bonds are chemical bonds that are covalent in nature and involve the lateral overlapping of two lobes of an atomic orbital with two lobes of another atomic orbital that belongs to a. Illustration of a pi bond forming from two p orbitals. Each of the two electrons in the pi bond (π. Pi Bonds Equation.
From www.numerade.com
SOLVED Identify the correct sequence of increasing number of π bonds Pi Bonds Equation Are pi bonds stronger than sigma bonds? Each of the two electrons in the pi bond (π bond) exists both above and below the plane of. Illustration of a pi bond forming from two p orbitals. The pi bond (π bond) has two halves—one above the plane of the molecule, and the other below it. Pi bonds are chemical bonds. Pi Bonds Equation.
From www.youtube.com
How to determine Sigma and Pi Bond Hybridization Organic Chemistry Pi Bonds Equation Each of the two electrons in the pi bond (π bond) exists both above and below the plane of. Pi bonds \((\pi)\) are a type of covalent bond formed by sideways or lateral overlapping of atomic orbitals. Notice how the orientation of. What is the difference between a sigma bond and a pi bond? Pi bonds are chemical bonds that. Pi Bonds Equation.
From www.youtube.com
How To Calculate Sigma And Pi Bond Super Trick to Find Sigma and Pi Pi Bonds Equation Notice how the orientation of. Pi bonds \((\pi)\) are a type of covalent bond formed by sideways or lateral overlapping of atomic orbitals. What is the difference between a sigma bond and a pi bond? Are pi bonds stronger than sigma bonds? Illustration of a pi bond forming from two p orbitals. The pi bond (π bond) has two halves—one. Pi Bonds Equation.
From www.drawittoknowit.com
Biochemistry Glossary Bonds 3. Pi Bonds Overlap Draw It to Know It Pi Bonds Equation Pi bonds \((\pi)\) are a type of covalent bond formed by sideways or lateral overlapping of atomic orbitals. Each of the two electrons in the pi bond (π bond) exists both above and below the plane of. Notice how the orientation of. Pi bonds are chemical bonds that are covalent in nature and involve the lateral overlapping of two lobes. Pi Bonds Equation.
From www.coursehero.com
[Solved] How many sigma and Pi bonds in this image? I'm not sure how Pi Bonds Equation The pi bond (π bond) has two halves—one above the plane of the molecule, and the other below it. Are pi bonds stronger than sigma bonds? Notice how the orientation of. Illustration of a pi bond forming from two p orbitals. Each of the two electrons in the pi bond (π bond) exists both above and below the plane of.. Pi Bonds Equation.
From www.doubtnut.com
Number of pi bonds and sigma bonds in the following structure is Pi Bonds Equation The pi bond (π bond) has two halves—one above the plane of the molecule, and the other below it. Pi bonds \((\pi)\) are a type of covalent bond formed by sideways or lateral overlapping of atomic orbitals. Are pi bonds stronger than sigma bonds? What is the difference between a sigma bond and a pi bond? Pi bonds are chemical. Pi Bonds Equation.
From sites.sandiego.edu
Ibuprofen Meet Your Medicine Pi Bonds Equation What is the difference between a sigma bond and a pi bond? Each of the two electrons in the pi bond (π bond) exists both above and below the plane of. Notice how the orientation of. Illustration of a pi bond forming from two p orbitals. Pi bonds are chemical bonds that are covalent in nature and involve the lateral. Pi Bonds Equation.
From www.youtube.com
Determining the number of Sigma and Pi Bonds in a Chemical Structure Pi Bonds Equation Pi bonds are chemical bonds that are covalent in nature and involve the lateral overlapping of two lobes of an atomic orbital with two lobes of another atomic orbital that belongs to a. Pi bonds \((\pi)\) are a type of covalent bond formed by sideways or lateral overlapping of atomic orbitals. Each of the two electrons in the pi bond. Pi Bonds Equation.
From www.slideserve.com
PPT Structural Analysis 3 PowerPoint Presentation, free download ID Pi Bonds Equation Each of the two electrons in the pi bond (π bond) exists both above and below the plane of. Pi bonds \((\pi)\) are a type of covalent bond formed by sideways or lateral overlapping of atomic orbitals. The pi bond (π bond) has two halves—one above the plane of the molecule, and the other below it. Pi bonds are chemical. Pi Bonds Equation.
From byjus.com
How many σ bonds and π bonds are present in the given compound?CH3 CH2 Pi Bonds Equation Notice how the orientation of. Pi bonds \((\pi)\) are a type of covalent bond formed by sideways or lateral overlapping of atomic orbitals. Pi bonds are chemical bonds that are covalent in nature and involve the lateral overlapping of two lobes of an atomic orbital with two lobes of another atomic orbital that belongs to a. The pi bond (π. Pi Bonds Equation.
From sekabusy.weebly.com
Ch2nn sigma and pi bonds sekabusy Pi Bonds Equation Notice how the orientation of. The pi bond (π bond) has two halves—one above the plane of the molecule, and the other below it. Illustration of a pi bond forming from two p orbitals. Are pi bonds stronger than sigma bonds? Pi bonds are chemical bonds that are covalent in nature and involve the lateral overlapping of two lobes of. Pi Bonds Equation.
From www.numerade.com
SOLVED Formula CH₂Cl2 Dichloromethane Lewis Electron Dot structure Pi Bonds Equation Pi bonds \((\pi)\) are a type of covalent bond formed by sideways or lateral overlapping of atomic orbitals. What is the difference between a sigma bond and a pi bond? Pi bonds are chemical bonds that are covalent in nature and involve the lateral overlapping of two lobes of an atomic orbital with two lobes of another atomic orbital that. Pi Bonds Equation.
From readingandwritingprojectcom.web.fc2.com
sigma bond pi bond difference Pi Bonds Equation Each of the two electrons in the pi bond (π bond) exists both above and below the plane of. Pi bonds are chemical bonds that are covalent in nature and involve the lateral overlapping of two lobes of an atomic orbital with two lobes of another atomic orbital that belongs to a. Are pi bonds stronger than sigma bonds? Illustration. Pi Bonds Equation.
From kaliyah-bogspotdiaz.blogspot.com
How to Find Pi Bonds in a Lewis Structure Pi Bonds Equation The pi bond (π bond) has two halves—one above the plane of the molecule, and the other below it. Each of the two electrons in the pi bond (π bond) exists both above and below the plane of. Are pi bonds stronger than sigma bonds? Pi bonds are chemical bonds that are covalent in nature and involve the lateral overlapping. Pi Bonds Equation.
From www.vedantu.com
How do you count sigma and pi bonds? For example, in the Lewis Pi Bonds Equation Each of the two electrons in the pi bond (π bond) exists both above and below the plane of. Notice how the orientation of. Illustration of a pi bond forming from two p orbitals. The pi bond (π bond) has two halves—one above the plane of the molecule, and the other below it. Pi bonds \((\pi)\) are a type of. Pi Bonds Equation.
From socratic.org
On average, how many pi bonds does "O"_2^(+) have? Socratic Pi Bonds Equation What is the difference between a sigma bond and a pi bond? Notice how the orientation of. Are pi bonds stronger than sigma bonds? Illustration of a pi bond forming from two p orbitals. Pi bonds are chemical bonds that are covalent in nature and involve the lateral overlapping of two lobes of an atomic orbital with two lobes of. Pi Bonds Equation.
From www.vedantu.com
How many sigma and pi bonds are in {{C}_{4}}{{H}_{6}} Pi Bonds Equation Illustration of a pi bond forming from two p orbitals. Pi bonds \((\pi)\) are a type of covalent bond formed by sideways or lateral overlapping of atomic orbitals. Are pi bonds stronger than sigma bonds? What is the difference between a sigma bond and a pi bond? Notice how the orientation of. Pi bonds are chemical bonds that are covalent. Pi Bonds Equation.