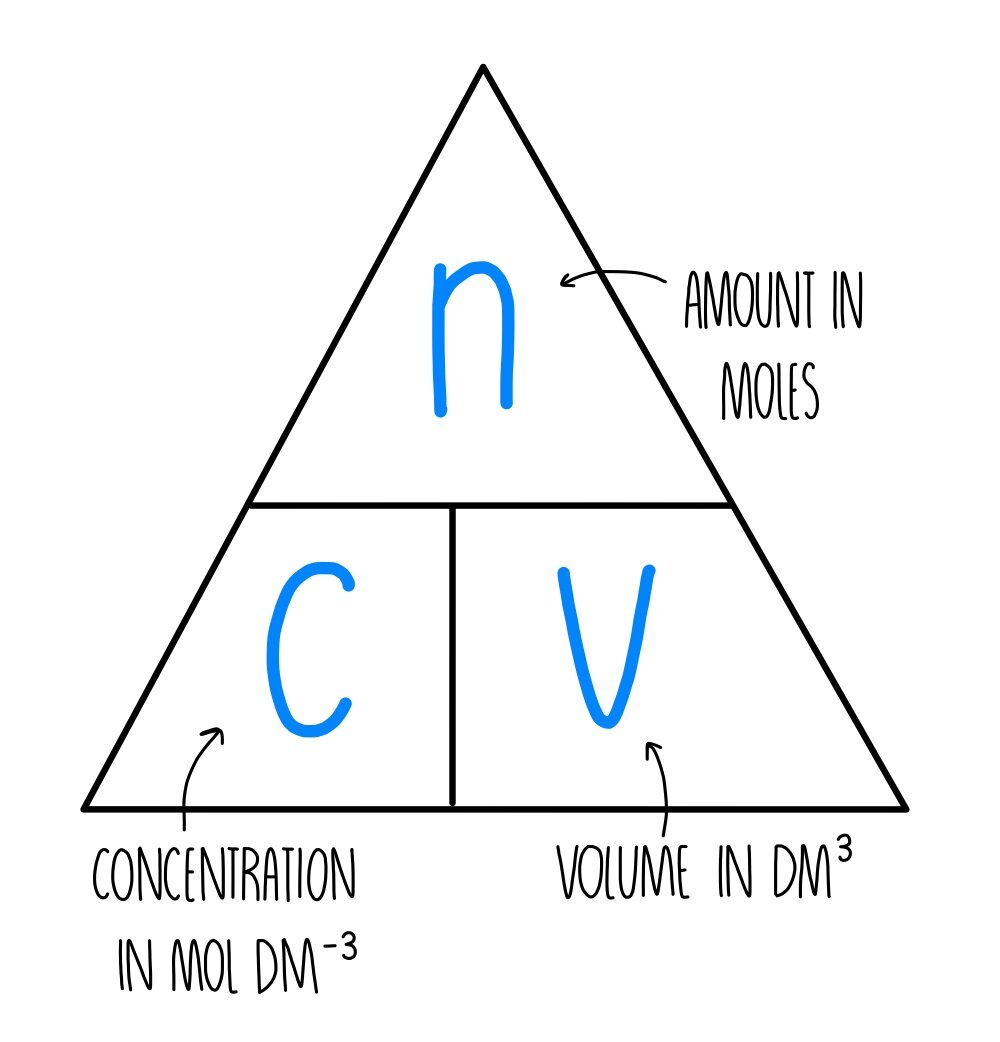
Titration Calculations Triangle . At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. The volumes of acids and alkali solutions that react with each other can be measured by. Aqa gcse chemistry chemical changes titration calculations. A titration is used to find an unknown concentration of a solution by reacting it with a solution of known concentration. Once a titration is completed, you can use the results to calculate. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. Formula triangle showing the relationship between concentration, number of moles and volume of liquid. The steps in a titration calculation are:.
from www.thesciencehive.co.uk
A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. The steps in a titration calculation are:. A titration is used to find an unknown concentration of a solution by reacting it with a solution of known concentration. Aqa gcse chemistry chemical changes titration calculations. The volumes of acids and alkali solutions that react with each other can be measured by. Once a titration is completed, you can use the results to calculate. Formula triangle showing the relationship between concentration, number of moles and volume of liquid.
Chemical formulae, equations and calculations GCSE — the science sauce
Titration Calculations Triangle The volumes of acids and alkali solutions that react with each other can be measured by. Formula triangle showing the relationship between concentration, number of moles and volume of liquid. A titration is used to find an unknown concentration of a solution by reacting it with a solution of known concentration. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. Aqa gcse chemistry chemical changes titration calculations. The steps in a titration calculation are:. The volumes of acids and alkali solutions that react with each other can be measured by. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. Once a titration is completed, you can use the results to calculate.
From www.vrogue.co
7 Inspiration Titration Calculations Worksheet Gcse M vrogue.co Titration Calculations Triangle A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. A titration is used to find an unknown concentration of a solution by reacting it with a solution of known concentration. Once a titration is completed, you can. Titration Calculations Triangle.
From kaytrinasofya.blogspot.com
37+ titration calculation questions gcse Titration Calculations Triangle Formula triangle showing the relationship between concentration, number of moles and volume of liquid. The steps in a titration calculation are:. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. A titration is used to find an unknown concentration of a solution by reacting it with a solution of known concentration.. Titration Calculations Triangle.
From www.youtube.com
Titration Calculations AQA GCSE Chemistry YouTube Titration Calculations Triangle Formula triangle showing the relationship between concentration, number of moles and volume of liquid. The volumes of acids and alkali solutions that react with each other can be measured by. Once a titration is completed, you can use the results to calculate. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base.. Titration Calculations Triangle.
From www.youtube.com
Titration Calculations, Paper 1+2 AQA A level Chemistry YouTube Titration Calculations Triangle The volumes of acids and alkali solutions that react with each other can be measured by. Once a titration is completed, you can use the results to calculate. A titration is used to find an unknown concentration of a solution by reacting it with a solution of known concentration. Aqa gcse chemistry chemical changes titration calculations. Formula triangle showing the. Titration Calculations Triangle.
From www.tes.com
4.7 Titration calculations Teaching Resources Titration Calculations Triangle Once a titration is completed, you can use the results to calculate. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. A titration is used to find an unknown concentration of a solution by reacting it with a solution of known concentration. The steps in a titration calculation are:. A titration. Titration Calculations Triangle.
From www.youtube.com
Titration Calculations National 5 Chemistry Lesson 5 YouTube Titration Calculations Triangle Once a titration is completed, you can use the results to calculate. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. Formula triangle showing the relationship between concentration, number of moles and volume of liquid. The steps in a titration calculation are:. A titration is a volumetric technique in which a. Titration Calculations Triangle.
From www.scribd.com
Titration Calculations Section A For these questions, your equation Titration Calculations Triangle The volumes of acids and alkali solutions that react with each other can be measured by. Formula triangle showing the relationship between concentration, number of moles and volume of liquid. Aqa gcse chemistry chemical changes titration calculations. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. A titration is used to. Titration Calculations Triangle.
From www.studocu.com
Chapter 14 Acid Base Titration Calculations in the context of Titration Calculations Triangle Aqa gcse chemistry chemical changes titration calculations. The volumes of acids and alkali solutions that react with each other can be measured by. Formula triangle showing the relationship between concentration, number of moles and volume of liquid. Once a titration is completed, you can use the results to calculate. The steps in a titration calculation are:. At the equivalence point. Titration Calculations Triangle.
From www.slideserve.com
PPT Titration calculations PowerPoint Presentation, free download Titration Calculations Triangle Formula triangle showing the relationship between concentration, number of moles and volume of liquid. A titration is used to find an unknown concentration of a solution by reacting it with a solution of known concentration. The volumes of acids and alkali solutions that react with each other can be measured by. A titration is a volumetric technique in which a. Titration Calculations Triangle.
From www.youtube.com
Titration Calculations YouTube Titration Calculations Triangle At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. The steps in a titration calculation are:. Once a titration. Titration Calculations Triangle.
From www.youtube.com
GCSE Chemistry Titration calculations worked examples YouTube Titration Calculations Triangle At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. Once a titration is completed, you can use the results to calculate. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point. Titration Calculations Triangle.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Titration Calculations Triangle Aqa gcse chemistry chemical changes titration calculations. The volumes of acids and alkali solutions that react with each other can be measured by. A titration is used to find an unknown concentration of a solution by reacting it with a solution of known concentration. The steps in a titration calculation are:. Once a titration is completed, you can use the. Titration Calculations Triangle.
From chem4three.blogspot.com
CHEMISTRY 11 TITRATIONS Titration Calculations Triangle A titration is used to find an unknown concentration of a solution by reacting it with a solution of known concentration. The volumes of acids and alkali solutions that react with each other can be measured by. Once a titration is completed, you can use the results to calculate. The steps in a titration calculation are:. A titration is a. Titration Calculations Triangle.
From www.youtube.com
Calculations with titrations 3 YouTube Titration Calculations Triangle A titration is used to find an unknown concentration of a solution by reacting it with a solution of known concentration. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. At the equivalence point in a neutralization,. Titration Calculations Triangle.
From thescienceteacher.co.uk
Concentration and titrations teaching resources the science teacher Titration Calculations Triangle Aqa gcse chemistry chemical changes titration calculations. Once a titration is completed, you can use the results to calculate. The steps in a titration calculation are:. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. A titration. Titration Calculations Triangle.
From www.youtube.com
OCR Gateway C5 AcidBase Titration Calculations (Higher) YouTube Titration Calculations Triangle A titration is used to find an unknown concentration of a solution by reacting it with a solution of known concentration. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. Formula triangle showing the relationship between concentration,. Titration Calculations Triangle.
From www.savemyexams.com
Titration Calculations Edexcel GCSE Chemistry Revision Notes 2018 Titration Calculations Triangle Once a titration is completed, you can use the results to calculate. The steps in a titration calculation are:. The volumes of acids and alkali solutions that react with each other can be measured by. Aqa gcse chemistry chemical changes titration calculations. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base.. Titration Calculations Triangle.
From ar.inspiredpencil.com
Titration Equation Titration Calculations Triangle Once a titration is completed, you can use the results to calculate. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. Formula triangle showing the relationship between concentration, number of moles and volume of liquid. The steps. Titration Calculations Triangle.
From mungfali.com
Acid Base Titration Calculation Titration Calculations Triangle Aqa gcse chemistry chemical changes titration calculations. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. A titration is used to find an unknown concentration of a solution by reacting it with a solution of known concentration.. Titration Calculations Triangle.
From www.youtube.com
Acid Base Titration Problems, Basic Introduction, Calculations Titration Calculations Triangle Aqa gcse chemistry chemical changes titration calculations. A titration is used to find an unknown concentration of a solution by reacting it with a solution of known concentration. Once a titration is completed, you can use the results to calculate. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution. Titration Calculations Triangle.
From www.studocu.com
Titration Calculations TITRATION CALCULATIONS WORKSHEET A 50 ml Titration Calculations Triangle At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. The steps in a titration calculation are:. Aqa gcse chemistry chemical changes titration calculations. Formula triangle showing the relationship between concentration, number of moles and volume of liquid. Once a titration is completed, you can use the results to calculate. A titration. Titration Calculations Triangle.
From www.youtube.com
How to Do Titration Calculations // HSC Chemistry YouTube Titration Calculations Triangle Formula triangle showing the relationship between concentration, number of moles and volume of liquid. Aqa gcse chemistry chemical changes titration calculations. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. The volumes of acids and alkali solutions. Titration Calculations Triangle.
From www.ck12.org
Titration (Calculations) Example 2 ( Video ) Chemistry CK12 Titration Calculations Triangle Aqa gcse chemistry chemical changes titration calculations. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. The volumes of acids and alkali solutions that react with each other can be measured by. At the equivalence point in. Titration Calculations Triangle.
From www.tes.com
Concentration and Titration Calculations GCSE Lesson (SC14c SC14d Titration Calculations Triangle A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. The volumes of acids and alkali solutions that react with each other can be measured by. At the equivalence point in a neutralization, the moles of acid are. Titration Calculations Triangle.
From mungfali.com
Acid Base Titration Procedure Titration Calculations Triangle Once a titration is completed, you can use the results to calculate. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. Formula triangle showing the relationship between concentration, number of moles and volume of liquid. A titration. Titration Calculations Triangle.
From www.youtube.com
Titration calculation example Chemistry Khan Academy YouTube Titration Calculations Triangle A titration is used to find an unknown concentration of a solution by reacting it with a solution of known concentration. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. Aqa gcse chemistry chemical changes titration calculations.. Titration Calculations Triangle.
From www.youtube.com
National 5 Titration calculations Triangle method YouTube Titration Calculations Triangle A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. Aqa gcse chemistry chemical changes titration calculations. Formula triangle showing the relationship between concentration, number of moles and volume of liquid. At the equivalence point in a neutralization,. Titration Calculations Triangle.
From missmeyerscience.blogspot.com
Miss Meyer's Science Site Titration Calculations Titration Calculations Triangle A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. Formula triangle showing the relationship between concentration, number of moles and volume of liquid. The steps in a titration calculation are:. The volumes of acids and alkali solutions. Titration Calculations Triangle.
From www.thesciencehive.co.uk
Chemical formulae, equations and calculations GCSE — the science sauce Titration Calculations Triangle Aqa gcse chemistry chemical changes titration calculations. Once a titration is completed, you can use the results to calculate. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second. Titration Calculations Triangle.
From moodle.tau.ac.il
AcidBase Titration Curves Titration Calculations Triangle Once a titration is completed, you can use the results to calculate. Formula triangle showing the relationship between concentration, number of moles and volume of liquid. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. The steps in a titration calculation are:. Aqa gcse chemistry chemical changes titration calculations. A titration. Titration Calculations Triangle.
From www.studocu.com
Titration Analysis of the health category CHAPTER 18 IV MEDICATION Titration Calculations Triangle A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. Once a titration is completed, you can use the results to calculate. The volumes of acids and alkali solutions that react with each other can be measured by.. Titration Calculations Triangle.
From www.slideserve.com
PPT Titration calculations PowerPoint Presentation, free download Titration Calculations Triangle Formula triangle showing the relationship between concentration, number of moles and volume of liquid. Once a titration is completed, you can use the results to calculate. The volumes of acids and alkali solutions that react with each other can be measured by. A titration is used to find an unknown concentration of a solution by reacting it with a solution. Titration Calculations Triangle.
From chem.libretexts.org
9.1 Overview of Titrimetry Chemistry LibreTexts Titration Calculations Triangle The steps in a titration calculation are:. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. Once a titration is completed, you can use the results to calculate. A titration is used to find an unknown concentration. Titration Calculations Triangle.
From www.ck12.org
Titration (Calculations) Example 3 ( Video ) Chemistry CK12 Titration Calculations Triangle The steps in a titration calculation are:. Aqa gcse chemistry chemical changes titration calculations. Once a titration is completed, you can use the results to calculate. Formula triangle showing the relationship between concentration, number of moles and volume of liquid. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution. Titration Calculations Triangle.
From slideplayer.com
triangle calculations ppt download Titration Calculations Triangle Once a titration is completed, you can use the results to calculate. The steps in a titration calculation are:. Formula triangle showing the relationship between concentration, number of moles and volume of liquid. The volumes of acids and alkali solutions that react with each other can be measured by. Aqa gcse chemistry chemical changes titration calculations. At the equivalence point. Titration Calculations Triangle.