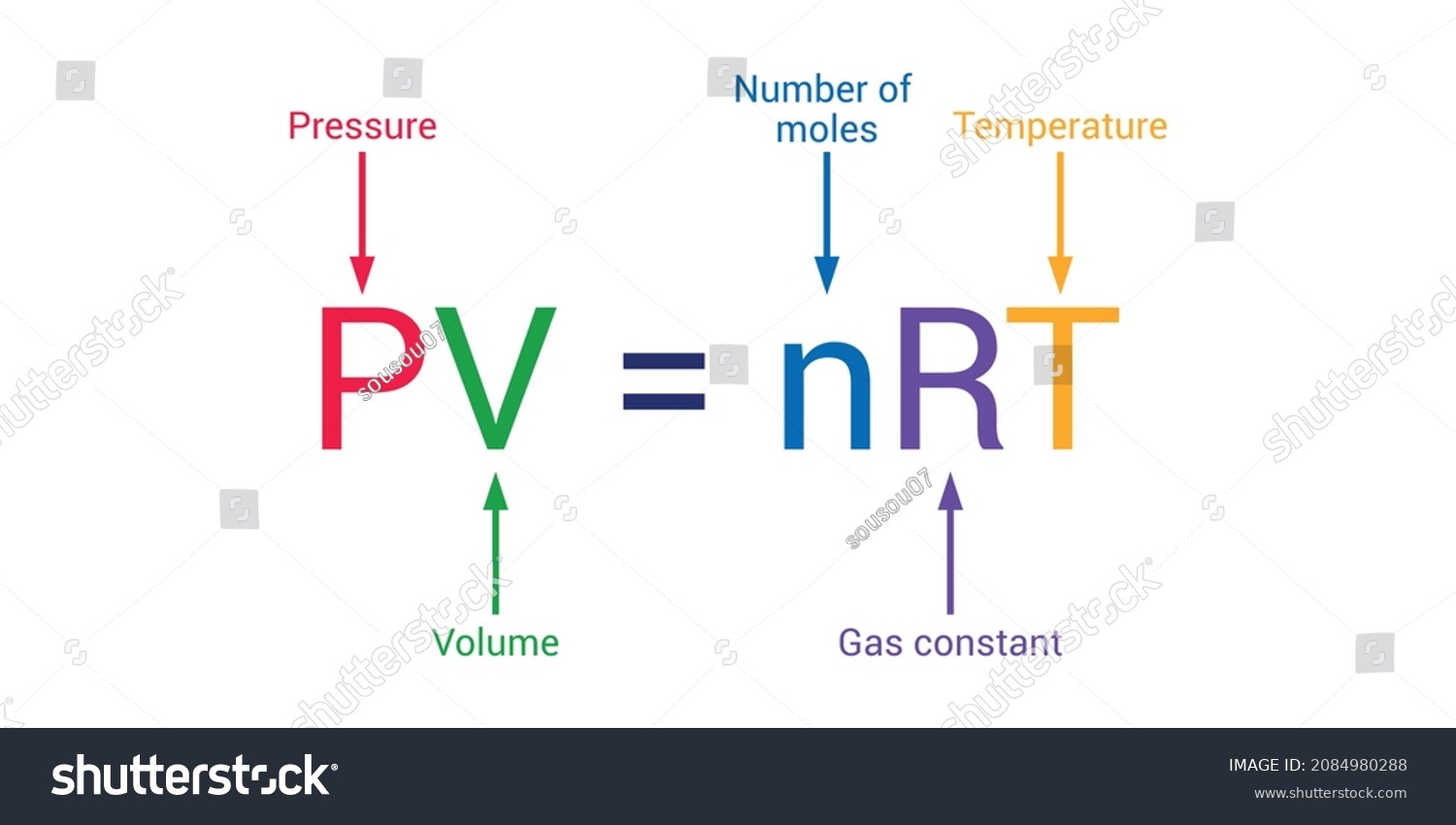
Gas With Formula . The ideal gas law relates the pressure and volume of a gas to the number of gas molecules and the temperature of the gas. The ideal gas law can be written in terms of the number of. Dalton's law of partial pressures. The ideal gas law is derived from empirical relationships among the pressure, the volume, the temperature, and the number of. Use the ideal gas equation to solve a problem when the amount of gas is given and the mass of the gas is constant. The ideal gas law can be. In such a case, all gases obey an equation of state known as the ideal gas law: Specifying any four of these terms will. Pv = nrt, where n is the number of moles of the gas and r is the universal (or perfect) gas. Kinetic theory of ideal gases (assumption for ideal gases) to describe an ideal. The ideal gas equation contains five terms, the gas constant r and the variable properties p, v, n, and t.
from www.shutterstock.com
The ideal gas equation contains five terms, the gas constant r and the variable properties p, v, n, and t. Specifying any four of these terms will. In such a case, all gases obey an equation of state known as the ideal gas law: Use the ideal gas equation to solve a problem when the amount of gas is given and the mass of the gas is constant. Kinetic theory of ideal gases (assumption for ideal gases) to describe an ideal. The ideal gas law can be written in terms of the number of. Pv = nrt, where n is the number of moles of the gas and r is the universal (or perfect) gas. The ideal gas law is derived from empirical relationships among the pressure, the volume, the temperature, and the number of. The ideal gas law relates the pressure and volume of a gas to the number of gas molecules and the temperature of the gas. Dalton's law of partial pressures.
Ideal Gas Law Formula Chemistry Stock Vector (Royalty Free) 2084980288
Gas With Formula The ideal gas equation contains five terms, the gas constant r and the variable properties p, v, n, and t. The ideal gas law is derived from empirical relationships among the pressure, the volume, the temperature, and the number of. In such a case, all gases obey an equation of state known as the ideal gas law: The ideal gas equation contains five terms, the gas constant r and the variable properties p, v, n, and t. Use the ideal gas equation to solve a problem when the amount of gas is given and the mass of the gas is constant. Dalton's law of partial pressures. The ideal gas law can be. Pv = nrt, where n is the number of moles of the gas and r is the universal (or perfect) gas. The ideal gas law relates the pressure and volume of a gas to the number of gas molecules and the temperature of the gas. Kinetic theory of ideal gases (assumption for ideal gases) to describe an ideal. Specifying any four of these terms will. The ideal gas law can be written in terms of the number of.
From mmerevise.co.uk
The Ideal Gas Equation MME Gas With Formula Specifying any four of these terms will. Pv = nrt, where n is the number of moles of the gas and r is the universal (or perfect) gas. The ideal gas law can be written in terms of the number of. The ideal gas equation contains five terms, the gas constant r and the variable properties p, v, n, and. Gas With Formula.
From korbin-well-orozco.blogspot.com
Formulas Used to Describe Gas Behavior Gas With Formula The ideal gas law can be. Dalton's law of partial pressures. In such a case, all gases obey an equation of state known as the ideal gas law: The ideal gas law is derived from empirical relationships among the pressure, the volume, the temperature, and the number of. Pv = nrt, where n is the number of moles of the. Gas With Formula.
From www.alamy.com
Ideal gas law, illustration Stock Photo Alamy Gas With Formula The ideal gas law is derived from empirical relationships among the pressure, the volume, the temperature, and the number of. Dalton's law of partial pressures. Kinetic theory of ideal gases (assumption for ideal gases) to describe an ideal. Pv = nrt, where n is the number of moles of the gas and r is the universal (or perfect) gas. The. Gas With Formula.
From en.ppt-online.org
The ideal gas equation online presentation Gas With Formula The ideal gas law relates the pressure and volume of a gas to the number of gas molecules and the temperature of the gas. The ideal gas equation contains five terms, the gas constant r and the variable properties p, v, n, and t. The ideal gas law can be written in terms of the number of. In such a. Gas With Formula.
From www.youtube.com
Thermodynamics 37 Ideal Gas Equation with compressibility factor Gas With Formula The ideal gas law can be. Pv = nrt, where n is the number of moles of the gas and r is the universal (or perfect) gas. In such a case, all gases obey an equation of state known as the ideal gas law: Use the ideal gas equation to solve a problem when the amount of gas is given. Gas With Formula.
From byjus.com
Different forms of ideal gas equation Gas With Formula Pv = nrt, where n is the number of moles of the gas and r is the universal (or perfect) gas. The ideal gas law can be. The ideal gas law can be written in terms of the number of. The ideal gas equation contains five terms, the gas constant r and the variable properties p, v, n, and t.. Gas With Formula.
From millingschem.com
Chemistry Resources 3 MillingsChem Gas With Formula Use the ideal gas equation to solve a problem when the amount of gas is given and the mass of the gas is constant. The ideal gas law is derived from empirical relationships among the pressure, the volume, the temperature, and the number of. In such a case, all gases obey an equation of state known as the ideal gas. Gas With Formula.
From www.slideserve.com
PPT 6 Gases PowerPoint Presentation ID352441 Gas With Formula Dalton's law of partial pressures. Specifying any four of these terms will. The ideal gas law can be. Pv = nrt, where n is the number of moles of the gas and r is the universal (or perfect) gas. In such a case, all gases obey an equation of state known as the ideal gas law: The ideal gas law. Gas With Formula.
From www.youtube.com
Volume of 1 mole of any gas at STP using the ideal gas equation YouTube Gas With Formula The ideal gas law can be written in terms of the number of. Pv = nrt, where n is the number of moles of the gas and r is the universal (or perfect) gas. Use the ideal gas equation to solve a problem when the amount of gas is given and the mass of the gas is constant. Specifying any. Gas With Formula.
From www.youtube.com
Ideal Gas Law Practice Problems YouTube Gas With Formula Pv = nrt, where n is the number of moles of the gas and r is the universal (or perfect) gas. Use the ideal gas equation to solve a problem when the amount of gas is given and the mass of the gas is constant. Dalton's law of partial pressures. The ideal gas law can be written in terms of. Gas With Formula.
From www.slideserve.com
PPT Combined Gas Law PowerPoint Presentation, free download ID5864985 Gas With Formula Dalton's law of partial pressures. Use the ideal gas equation to solve a problem when the amount of gas is given and the mass of the gas is constant. The ideal gas law can be. Pv = nrt, where n is the number of moles of the gas and r is the universal (or perfect) gas. The ideal gas law. Gas With Formula.
From www.showme.com
Density from Ideal gas equation Chemistry, Science, Gases ShowMe Gas With Formula The ideal gas equation contains five terms, the gas constant r and the variable properties p, v, n, and t. The ideal gas law is derived from empirical relationships among the pressure, the volume, the temperature, and the number of. The ideal gas law relates the pressure and volume of a gas to the number of gas molecules and the. Gas With Formula.
From www.slideserve.com
PPT The General Gas Equation Combined Gas Law PowerPoint Presentation Gas With Formula Use the ideal gas equation to solve a problem when the amount of gas is given and the mass of the gas is constant. The ideal gas law is derived from empirical relationships among the pressure, the volume, the temperature, and the number of. Kinetic theory of ideal gases (assumption for ideal gases) to describe an ideal. The ideal gas. Gas With Formula.
From www.youtube.com
Mass and volume flow rates Ideal Gas Example YouTube Gas With Formula Kinetic theory of ideal gases (assumption for ideal gases) to describe an ideal. The ideal gas law relates the pressure and volume of a gas to the number of gas molecules and the temperature of the gas. The ideal gas law is derived from empirical relationships among the pressure, the volume, the temperature, and the number of. The ideal gas. Gas With Formula.
From sciencenotes.org
Combined Gas Law Definition, Formula, Examples Gas With Formula Pv = nrt, where n is the number of moles of the gas and r is the universal (or perfect) gas. The ideal gas law can be written in terms of the number of. The ideal gas law relates the pressure and volume of a gas to the number of gas molecules and the temperature of the gas. Kinetic theory. Gas With Formula.
From www.youtube.com
In the ideal gas equation, the gas constant `R` has the dimension of Gas With Formula Kinetic theory of ideal gases (assumption for ideal gases) to describe an ideal. In such a case, all gases obey an equation of state known as the ideal gas law: The ideal gas law can be. Pv = nrt, where n is the number of moles of the gas and r is the universal (or perfect) gas. The ideal gas. Gas With Formula.
From www.slideserve.com
PPT IdealGas Equation PowerPoint Presentation, free download ID Gas With Formula The ideal gas law can be written in terms of the number of. The ideal gas law is derived from empirical relationships among the pressure, the volume, the temperature, and the number of. The ideal gas law can be. Kinetic theory of ideal gases (assumption for ideal gases) to describe an ideal. The ideal gas equation contains five terms, the. Gas With Formula.
From chemistry101efhs.weebly.com
Gas Laws Chemistry 101 Gas With Formula The ideal gas equation contains five terms, the gas constant r and the variable properties p, v, n, and t. Dalton's law of partial pressures. Specifying any four of these terms will. In such a case, all gases obey an equation of state known as the ideal gas law: The ideal gas law is derived from empirical relationships among the. Gas With Formula.
From www.youtube.com
Gas Law Formulas and Equations College Chemistry Study Guide YouTube Gas With Formula Dalton's law of partial pressures. Use the ideal gas equation to solve a problem when the amount of gas is given and the mass of the gas is constant. Pv = nrt, where n is the number of moles of the gas and r is the universal (or perfect) gas. Specifying any four of these terms will. The ideal gas. Gas With Formula.
From chem-net.blogspot.com
Gas Laws Ideal Gas Law Chemistry Net Gas With Formula Kinetic theory of ideal gases (assumption for ideal gases) to describe an ideal. The ideal gas law is derived from empirical relationships among the pressure, the volume, the temperature, and the number of. Specifying any four of these terms will. The ideal gas law relates the pressure and volume of a gas to the number of gas molecules and the. Gas With Formula.
From www.slideserve.com
PPT The Ideal Gas Equation PowerPoint Presentation, free download Gas With Formula The ideal gas law can be. The ideal gas law is derived from empirical relationships among the pressure, the volume, the temperature, and the number of. Kinetic theory of ideal gases (assumption for ideal gases) to describe an ideal. Specifying any four of these terms will. Use the ideal gas equation to solve a problem when the amount of gas. Gas With Formula.
From www.slideserve.com
PPT The Ideal Gas Equation PowerPoint Presentation, free download Gas With Formula The ideal gas equation contains five terms, the gas constant r and the variable properties p, v, n, and t. In such a case, all gases obey an equation of state known as the ideal gas law: Dalton's law of partial pressures. Pv = nrt, where n is the number of moles of the gas and r is the universal. Gas With Formula.
From www.youtube.com
Molar Mass of a Gas at STP Equations & Formulas, Chemistry Practice Gas With Formula The ideal gas law can be. The ideal gas law can be written in terms of the number of. The ideal gas law relates the pressure and volume of a gas to the number of gas molecules and the temperature of the gas. Dalton's law of partial pressures. The ideal gas law is derived from empirical relationships among the pressure,. Gas With Formula.
From www.slideserve.com
PPT IdealGas Equation PowerPoint Presentation, free download ID Gas With Formula The ideal gas law can be. Use the ideal gas equation to solve a problem when the amount of gas is given and the mass of the gas is constant. Kinetic theory of ideal gases (assumption for ideal gases) to describe an ideal. The ideal gas law is derived from empirical relationships among the pressure, the volume, the temperature, and. Gas With Formula.
From www.shutterstock.com
Ideal Gas Law Formula Chemistry Stock Vector (Royalty Free) 2084980288 Gas With Formula In such a case, all gases obey an equation of state known as the ideal gas law: Specifying any four of these terms will. The ideal gas equation contains five terms, the gas constant r and the variable properties p, v, n, and t. Use the ideal gas equation to solve a problem when the amount of gas is given. Gas With Formula.
From www.slideserve.com
PPT IdealGas Equation PowerPoint Presentation, free download ID Gas With Formula In such a case, all gases obey an equation of state known as the ideal gas law: The ideal gas law can be written in terms of the number of. Dalton's law of partial pressures. The ideal gas law is derived from empirical relationships among the pressure, the volume, the temperature, and the number of. Pv = nrt, where n. Gas With Formula.
From chemistryskills.com
Ideal Gas Law Combined Gas Law Chemistry Skills Gas With Formula Specifying any four of these terms will. Pv = nrt, where n is the number of moles of the gas and r is the universal (or perfect) gas. The ideal gas equation contains five terms, the gas constant r and the variable properties p, v, n, and t. The ideal gas law can be written in terms of the number. Gas With Formula.
From www.slideserve.com
PPT Ideal Gas Equation PowerPoint Presentation, free download ID Gas With Formula The ideal gas law can be written in terms of the number of. Specifying any four of these terms will. Kinetic theory of ideal gases (assumption for ideal gases) to describe an ideal. Use the ideal gas equation to solve a problem when the amount of gas is given and the mass of the gas is constant. Pv = nrt,. Gas With Formula.
From www.youtube.com
1.3 Ideal gas equation YouTube Gas With Formula Kinetic theory of ideal gases (assumption for ideal gases) to describe an ideal. The ideal gas law is derived from empirical relationships among the pressure, the volume, the temperature, and the number of. Pv = nrt, where n is the number of moles of the gas and r is the universal (or perfect) gas. The ideal gas equation contains five. Gas With Formula.
From www.youtube.com
Combined Gas Law Formula (Chemistry) YouTube Gas With Formula The ideal gas law can be. The ideal gas law is derived from empirical relationships among the pressure, the volume, the temperature, and the number of. Specifying any four of these terms will. Use the ideal gas equation to solve a problem when the amount of gas is given and the mass of the gas is constant. Dalton's law of. Gas With Formula.
From sciencenotes.org
Ideal Gas Law Formula and Examples Gas With Formula The ideal gas law relates the pressure and volume of a gas to the number of gas molecules and the temperature of the gas. The ideal gas law can be. The ideal gas law can be written in terms of the number of. The ideal gas law is derived from empirical relationships among the pressure, the volume, the temperature, and. Gas With Formula.
From www.slideserve.com
PPT The General Gas Equation Combined Gas Law PowerPoint Presentation Gas With Formula In such a case, all gases obey an equation of state known as the ideal gas law: Use the ideal gas equation to solve a problem when the amount of gas is given and the mass of the gas is constant. The ideal gas equation contains five terms, the gas constant r and the variable properties p, v, n, and. Gas With Formula.
From www.slideserve.com
PPT Ideal Gas Equation PowerPoint Presentation, free download ID Gas With Formula The ideal gas law relates the pressure and volume of a gas to the number of gas molecules and the temperature of the gas. Kinetic theory of ideal gases (assumption for ideal gases) to describe an ideal. The ideal gas law can be written in terms of the number of. Use the ideal gas equation to solve a problem when. Gas With Formula.
From byjus.com
Ideal Gas Law Equation Compressibility Of Natural Gas Chemistry Gas With Formula Pv = nrt, where n is the number of moles of the gas and r is the universal (or perfect) gas. The ideal gas law is derived from empirical relationships among the pressure, the volume, the temperature, and the number of. Dalton's law of partial pressures. The ideal gas law relates the pressure and volume of a gas to the. Gas With Formula.
From www.youtube.com
Empirical/Molecular formula with the Ideal Gas Law Chemistry Sample Gas With Formula The ideal gas equation contains five terms, the gas constant r and the variable properties p, v, n, and t. Use the ideal gas equation to solve a problem when the amount of gas is given and the mass of the gas is constant. The ideal gas law relates the pressure and volume of a gas to the number of. Gas With Formula.