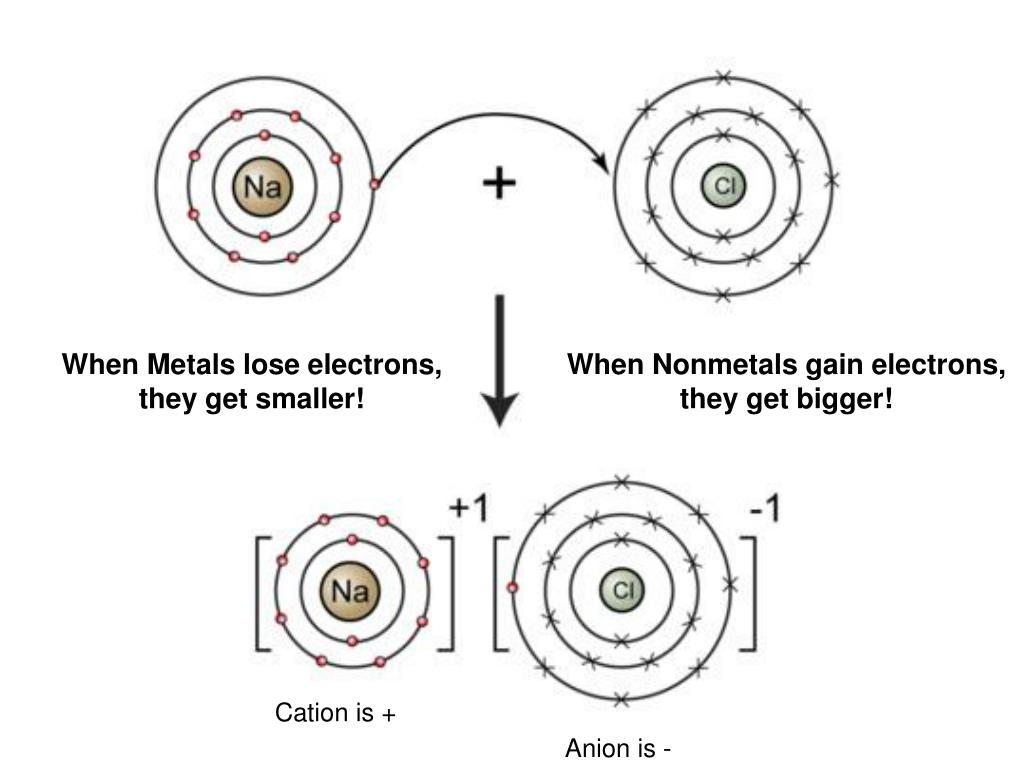
Why Are Ionic Compounds Formed With Metals And Nonmetals . When the two ions combine via ionic bond, they form ionic compounds. Molecular compounds form between nonmetals and nonmetals, while ionic compounds form between metals and nonmetals. We'll explain how they show trends in their physical. The key difference between an ionic and covalent bond is that one atom essentially donates an electron to another atom in an ionic bond while electrons are shared between. Discover how metals and nonmetals interact to form strong ionic bonds 🔗 by transferring. How do ionic bonds form. By definition, a metal is relatively stable if it loses electrons to form a complete valence shell and. In other words, ionic compounds are held together by ionic bonds are classed as ionic compounds.
from www.slideserve.com
We'll explain how they show trends in their physical. In other words, ionic compounds are held together by ionic bonds are classed as ionic compounds. How do ionic bonds form. Molecular compounds form between nonmetals and nonmetals, while ionic compounds form between metals and nonmetals. By definition, a metal is relatively stable if it loses electrons to form a complete valence shell and. When the two ions combine via ionic bond, they form ionic compounds. Discover how metals and nonmetals interact to form strong ionic bonds 🔗 by transferring. The key difference between an ionic and covalent bond is that one atom essentially donates an electron to another atom in an ionic bond while electrons are shared between.
PPT Ionic and Metallic Bonding PowerPoint Presentation, free download
Why Are Ionic Compounds Formed With Metals And Nonmetals Molecular compounds form between nonmetals and nonmetals, while ionic compounds form between metals and nonmetals. When the two ions combine via ionic bond, they form ionic compounds. How do ionic bonds form. Discover how metals and nonmetals interact to form strong ionic bonds 🔗 by transferring. The key difference between an ionic and covalent bond is that one atom essentially donates an electron to another atom in an ionic bond while electrons are shared between. Molecular compounds form between nonmetals and nonmetals, while ionic compounds form between metals and nonmetals. In other words, ionic compounds are held together by ionic bonds are classed as ionic compounds. We'll explain how they show trends in their physical. By definition, a metal is relatively stable if it loses electrons to form a complete valence shell and.
From www.numerade.com
SOLVED Name five metals and five nonmetals that are very likely to Why Are Ionic Compounds Formed With Metals And Nonmetals By definition, a metal is relatively stable if it loses electrons to form a complete valence shell and. Discover how metals and nonmetals interact to form strong ionic bonds 🔗 by transferring. The key difference between an ionic and covalent bond is that one atom essentially donates an electron to another atom in an ionic bond while electrons are shared. Why Are Ionic Compounds Formed With Metals And Nonmetals.
From jaylenyouthmejia.blogspot.com
Ionic Bonding Between Metals and Nonmetals Why Are Ionic Compounds Formed With Metals And Nonmetals Molecular compounds form between nonmetals and nonmetals, while ionic compounds form between metals and nonmetals. By definition, a metal is relatively stable if it loses electrons to form a complete valence shell and. When the two ions combine via ionic bond, they form ionic compounds. Discover how metals and nonmetals interact to form strong ionic bonds 🔗 by transferring. We'll. Why Are Ionic Compounds Formed With Metals And Nonmetals.
From slideplayer.com
Chemical Bonds. ppt download Why Are Ionic Compounds Formed With Metals And Nonmetals In other words, ionic compounds are held together by ionic bonds are classed as ionic compounds. When the two ions combine via ionic bond, they form ionic compounds. Discover how metals and nonmetals interact to form strong ionic bonds 🔗 by transferring. How do ionic bonds form. We'll explain how they show trends in their physical. Molecular compounds form between. Why Are Ionic Compounds Formed With Metals And Nonmetals.
From slideplayer.com
Chapter 7 Periodic Properties of the Elements ppt download Why Are Ionic Compounds Formed With Metals And Nonmetals We'll explain how they show trends in their physical. Molecular compounds form between nonmetals and nonmetals, while ionic compounds form between metals and nonmetals. The key difference between an ionic and covalent bond is that one atom essentially donates an electron to another atom in an ionic bond while electrons are shared between. Discover how metals and nonmetals interact to. Why Are Ionic Compounds Formed With Metals And Nonmetals.
From www.numerade.com
SOLVED If energy is required to form monatomic ions from metals and Why Are Ionic Compounds Formed With Metals And Nonmetals The key difference between an ionic and covalent bond is that one atom essentially donates an electron to another atom in an ionic bond while electrons are shared between. We'll explain how they show trends in their physical. Molecular compounds form between nonmetals and nonmetals, while ionic compounds form between metals and nonmetals. How do ionic bonds form. By definition,. Why Are Ionic Compounds Formed With Metals And Nonmetals.
From www.slideserve.com
PPT Ionic and Metallic Bonding PowerPoint Presentation, free download Why Are Ionic Compounds Formed With Metals And Nonmetals We'll explain how they show trends in their physical. In other words, ionic compounds are held together by ionic bonds are classed as ionic compounds. Discover how metals and nonmetals interact to form strong ionic bonds 🔗 by transferring. By definition, a metal is relatively stable if it loses electrons to form a complete valence shell and. How do ionic. Why Are Ionic Compounds Formed With Metals And Nonmetals.
From slidetodoc.com
Metals Compounds formed between metals and nonmetals tend Why Are Ionic Compounds Formed With Metals And Nonmetals Discover how metals and nonmetals interact to form strong ionic bonds 🔗 by transferring. How do ionic bonds form. When the two ions combine via ionic bond, they form ionic compounds. We'll explain how they show trends in their physical. Molecular compounds form between nonmetals and nonmetals, while ionic compounds form between metals and nonmetals. The key difference between an. Why Are Ionic Compounds Formed With Metals And Nonmetals.
From slideplayer.com
Ionic Bonding and Naming ppt download Why Are Ionic Compounds Formed With Metals And Nonmetals When the two ions combine via ionic bond, they form ionic compounds. By definition, a metal is relatively stable if it loses electrons to form a complete valence shell and. We'll explain how they show trends in their physical. Discover how metals and nonmetals interact to form strong ionic bonds 🔗 by transferring. In other words, ionic compounds are held. Why Are Ionic Compounds Formed With Metals And Nonmetals.
From www.youtube.com
Ionic bonds Reaction of metals & Nonmetals Metals and non metals Why Are Ionic Compounds Formed With Metals And Nonmetals Molecular compounds form between nonmetals and nonmetals, while ionic compounds form between metals and nonmetals. We'll explain how they show trends in their physical. In other words, ionic compounds are held together by ionic bonds are classed as ionic compounds. The key difference between an ionic and covalent bond is that one atom essentially donates an electron to another atom. Why Are Ionic Compounds Formed With Metals And Nonmetals.
From www.youtube.com
Examples of Ionic Bonding YouTube Why Are Ionic Compounds Formed With Metals And Nonmetals When the two ions combine via ionic bond, they form ionic compounds. Molecular compounds form between nonmetals and nonmetals, while ionic compounds form between metals and nonmetals. In other words, ionic compounds are held together by ionic bonds are classed as ionic compounds. How do ionic bonds form. The key difference between an ionic and covalent bond is that one. Why Are Ionic Compounds Formed With Metals And Nonmetals.
From www.slideserve.com
PPT NonMetals PowerPoint Presentation, free download ID2409577 Why Are Ionic Compounds Formed With Metals And Nonmetals Discover how metals and nonmetals interact to form strong ionic bonds 🔗 by transferring. We'll explain how they show trends in their physical. By definition, a metal is relatively stable if it loses electrons to form a complete valence shell and. Molecular compounds form between nonmetals and nonmetals, while ionic compounds form between metals and nonmetals. In other words, ionic. Why Are Ionic Compounds Formed With Metals And Nonmetals.
From slideplayer.com
Chapter 2 Atoms, Molecules, and Ions ppt download Why Are Ionic Compounds Formed With Metals And Nonmetals How do ionic bonds form. In other words, ionic compounds are held together by ionic bonds are classed as ionic compounds. Discover how metals and nonmetals interact to form strong ionic bonds 🔗 by transferring. When the two ions combine via ionic bond, they form ionic compounds. The key difference between an ionic and covalent bond is that one atom. Why Are Ionic Compounds Formed With Metals And Nonmetals.
From slideplayer.com
Atoms, Molecules, and Ions © 2009, PrenticeHall, Inc. Ionic Bonds Why Are Ionic Compounds Formed With Metals And Nonmetals By definition, a metal is relatively stable if it loses electrons to form a complete valence shell and. The key difference between an ionic and covalent bond is that one atom essentially donates an electron to another atom in an ionic bond while electrons are shared between. Discover how metals and nonmetals interact to form strong ionic bonds 🔗 by. Why Are Ionic Compounds Formed With Metals And Nonmetals.
From www.slideserve.com
PPT Ions, Ionic Nomenclature, Molecular Compounds, and Metals Why Are Ionic Compounds Formed With Metals And Nonmetals We'll explain how they show trends in their physical. In other words, ionic compounds are held together by ionic bonds are classed as ionic compounds. When the two ions combine via ionic bond, they form ionic compounds. The key difference between an ionic and covalent bond is that one atom essentially donates an electron to another atom in an ionic. Why Are Ionic Compounds Formed With Metals And Nonmetals.
From derekcarrsavvy-chemist.blogspot.com
savvychemist GCSE OCR Gateway Chemistry C2.2 ac Metals and nonmetals Why Are Ionic Compounds Formed With Metals And Nonmetals By definition, a metal is relatively stable if it loses electrons to form a complete valence shell and. We'll explain how they show trends in their physical. Discover how metals and nonmetals interact to form strong ionic bonds 🔗 by transferring. How do ionic bonds form. In other words, ionic compounds are held together by ionic bonds are classed as. Why Are Ionic Compounds Formed With Metals And Nonmetals.
From www.slideserve.com
PPT NAMING COMPOUNDS PowerPoint Presentation, free download ID3760730 Why Are Ionic Compounds Formed With Metals And Nonmetals We'll explain how they show trends in their physical. When the two ions combine via ionic bond, they form ionic compounds. How do ionic bonds form. Molecular compounds form between nonmetals and nonmetals, while ionic compounds form between metals and nonmetals. In other words, ionic compounds are held together by ionic bonds are classed as ionic compounds. Discover how metals. Why Are Ionic Compounds Formed With Metals And Nonmetals.
From en.wikipedia.org
Ionic bonding Wikipedia Why Are Ionic Compounds Formed With Metals And Nonmetals In other words, ionic compounds are held together by ionic bonds are classed as ionic compounds. When the two ions combine via ionic bond, they form ionic compounds. Discover how metals and nonmetals interact to form strong ionic bonds 🔗 by transferring. By definition, a metal is relatively stable if it loses electrons to form a complete valence shell and.. Why Are Ionic Compounds Formed With Metals And Nonmetals.
From www.britannica.com
Ionic bond Definition, Properties, Examples, & Facts Britannica Why Are Ionic Compounds Formed With Metals And Nonmetals How do ionic bonds form. In other words, ionic compounds are held together by ionic bonds are classed as ionic compounds. By definition, a metal is relatively stable if it loses electrons to form a complete valence shell and. Discover how metals and nonmetals interact to form strong ionic bonds 🔗 by transferring. When the two ions combine via ionic. Why Are Ionic Compounds Formed With Metals And Nonmetals.
From www.stonecoldhands.com
Ionic Compounds Stone Cold Chemistry Talk Why Are Ionic Compounds Formed With Metals And Nonmetals Discover how metals and nonmetals interact to form strong ionic bonds 🔗 by transferring. The key difference between an ionic and covalent bond is that one atom essentially donates an electron to another atom in an ionic bond while electrons are shared between. Molecular compounds form between nonmetals and nonmetals, while ionic compounds form between metals and nonmetals. In other. Why Are Ionic Compounds Formed With Metals And Nonmetals.
From www.slideserve.com
PPT Ionic Compounds Formula to Name PowerPoint Presentation, free Why Are Ionic Compounds Formed With Metals And Nonmetals When the two ions combine via ionic bond, they form ionic compounds. Discover how metals and nonmetals interact to form strong ionic bonds 🔗 by transferring. By definition, a metal is relatively stable if it loses electrons to form a complete valence shell and. Molecular compounds form between nonmetals and nonmetals, while ionic compounds form between metals and nonmetals. We'll. Why Are Ionic Compounds Formed With Metals And Nonmetals.
From slideplayer.com
Chapter 7 Periodic Properties of the Elements ppt download Why Are Ionic Compounds Formed With Metals And Nonmetals The key difference between an ionic and covalent bond is that one atom essentially donates an electron to another atom in an ionic bond while electrons are shared between. By definition, a metal is relatively stable if it loses electrons to form a complete valence shell and. Molecular compounds form between nonmetals and nonmetals, while ionic compounds form between metals. Why Are Ionic Compounds Formed With Metals And Nonmetals.
From jaylenyouthmejia.blogspot.com
Ionic Bonding Between Metals and Nonmetals Why Are Ionic Compounds Formed With Metals And Nonmetals Discover how metals and nonmetals interact to form strong ionic bonds 🔗 by transferring. How do ionic bonds form. We'll explain how they show trends in their physical. When the two ions combine via ionic bond, they form ionic compounds. By definition, a metal is relatively stable if it loses electrons to form a complete valence shell and. In other. Why Are Ionic Compounds Formed With Metals And Nonmetals.
From www.slideserve.com
PPT Molecular Compounds PowerPoint Presentation, free download ID Why Are Ionic Compounds Formed With Metals And Nonmetals The key difference between an ionic and covalent bond is that one atom essentially donates an electron to another atom in an ionic bond while electrons are shared between. When the two ions combine via ionic bond, they form ionic compounds. Discover how metals and nonmetals interact to form strong ionic bonds 🔗 by transferring. By definition, a metal is. Why Are Ionic Compounds Formed With Metals And Nonmetals.
From www.slideserve.com
PPT QUICK REVIEW OF ELEMENTS , IONS , and COMPOUNDS PowerPoint Why Are Ionic Compounds Formed With Metals And Nonmetals We'll explain how they show trends in their physical. When the two ions combine via ionic bond, they form ionic compounds. Discover how metals and nonmetals interact to form strong ionic bonds 🔗 by transferring. In other words, ionic compounds are held together by ionic bonds are classed as ionic compounds. How do ionic bonds form. By definition, a metal. Why Are Ionic Compounds Formed With Metals And Nonmetals.
From www.slideserve.com
PPT Ionic Compounds and Metals PowerPoint Presentation, free download Why Are Ionic Compounds Formed With Metals And Nonmetals In other words, ionic compounds are held together by ionic bonds are classed as ionic compounds. Discover how metals and nonmetals interact to form strong ionic bonds 🔗 by transferring. When the two ions combine via ionic bond, they form ionic compounds. How do ionic bonds form. The key difference between an ionic and covalent bond is that one atom. Why Are Ionic Compounds Formed With Metals And Nonmetals.
From www.jagranjosh.com
What are Ionic Compounds and how they are formed? Why Are Ionic Compounds Formed With Metals And Nonmetals By definition, a metal is relatively stable if it loses electrons to form a complete valence shell and. Discover how metals and nonmetals interact to form strong ionic bonds 🔗 by transferring. How do ionic bonds form. When the two ions combine via ionic bond, they form ionic compounds. The key difference between an ionic and covalent bond is that. Why Are Ionic Compounds Formed With Metals And Nonmetals.
From slideplayer.com
Ionic and Covalent Compounds ppt download Why Are Ionic Compounds Formed With Metals And Nonmetals How do ionic bonds form. By definition, a metal is relatively stable if it loses electrons to form a complete valence shell and. When the two ions combine via ionic bond, they form ionic compounds. Discover how metals and nonmetals interact to form strong ionic bonds 🔗 by transferring. We'll explain how they show trends in their physical. In other. Why Are Ionic Compounds Formed With Metals And Nonmetals.
From www.slideserve.com
PPT Naming Chemical Formulas PowerPoint Presentation ID4196035 Why Are Ionic Compounds Formed With Metals And Nonmetals In other words, ionic compounds are held together by ionic bonds are classed as ionic compounds. When the two ions combine via ionic bond, they form ionic compounds. Discover how metals and nonmetals interact to form strong ionic bonds 🔗 by transferring. The key difference between an ionic and covalent bond is that one atom essentially donates an electron to. Why Are Ionic Compounds Formed With Metals And Nonmetals.
From www.shalom-education.com
Properties of Ionic Compounds GCSE Chemistry Revision Why Are Ionic Compounds Formed With Metals And Nonmetals Discover how metals and nonmetals interact to form strong ionic bonds 🔗 by transferring. The key difference between an ionic and covalent bond is that one atom essentially donates an electron to another atom in an ionic bond while electrons are shared between. By definition, a metal is relatively stable if it loses electrons to form a complete valence shell. Why Are Ionic Compounds Formed With Metals And Nonmetals.
From www.sliderbase.com
Ionic Bonding Presentation Chemistry Why Are Ionic Compounds Formed With Metals And Nonmetals We'll explain how they show trends in their physical. By definition, a metal is relatively stable if it loses electrons to form a complete valence shell and. Discover how metals and nonmetals interact to form strong ionic bonds 🔗 by transferring. In other words, ionic compounds are held together by ionic bonds are classed as ionic compounds. The key difference. Why Are Ionic Compounds Formed With Metals And Nonmetals.
From www.youtube.com
why do ionic compounds have high melting points metals and non metals Why Are Ionic Compounds Formed With Metals And Nonmetals How do ionic bonds form. Molecular compounds form between nonmetals and nonmetals, while ionic compounds form between metals and nonmetals. Discover how metals and nonmetals interact to form strong ionic bonds 🔗 by transferring. We'll explain how they show trends in their physical. The key difference between an ionic and covalent bond is that one atom essentially donates an electron. Why Are Ionic Compounds Formed With Metals And Nonmetals.
From shareeducatonideas.com
What Is An Ionic Compound? Formula and Defination Why Are Ionic Compounds Formed With Metals And Nonmetals We'll explain how they show trends in their physical. In other words, ionic compounds are held together by ionic bonds are classed as ionic compounds. Molecular compounds form between nonmetals and nonmetals, while ionic compounds form between metals and nonmetals. How do ionic bonds form. Discover how metals and nonmetals interact to form strong ionic bonds 🔗 by transferring. When. Why Are Ionic Compounds Formed With Metals And Nonmetals.
From www.bartleby.com
Metals and Nonmetals bartleby Why Are Ionic Compounds Formed With Metals And Nonmetals The key difference between an ionic and covalent bond is that one atom essentially donates an electron to another atom in an ionic bond while electrons are shared between. By definition, a metal is relatively stable if it loses electrons to form a complete valence shell and. How do ionic bonds form. Molecular compounds form between nonmetals and nonmetals, while. Why Are Ionic Compounds Formed With Metals And Nonmetals.
From www.slideserve.com
PPT Ions, Ionic Nomenclature, Molecular Compounds, and Metals Why Are Ionic Compounds Formed With Metals And Nonmetals We'll explain how they show trends in their physical. In other words, ionic compounds are held together by ionic bonds are classed as ionic compounds. Molecular compounds form between nonmetals and nonmetals, while ionic compounds form between metals and nonmetals. The key difference between an ionic and covalent bond is that one atom essentially donates an electron to another atom. Why Are Ionic Compounds Formed With Metals And Nonmetals.
From www.nagwa.com
Question Video Determining Which Two Types of Elements Form an Ionic Why Are Ionic Compounds Formed With Metals And Nonmetals When the two ions combine via ionic bond, they form ionic compounds. Discover how metals and nonmetals interact to form strong ionic bonds 🔗 by transferring. The key difference between an ionic and covalent bond is that one atom essentially donates an electron to another atom in an ionic bond while electrons are shared between. By definition, a metal is. Why Are Ionic Compounds Formed With Metals And Nonmetals.