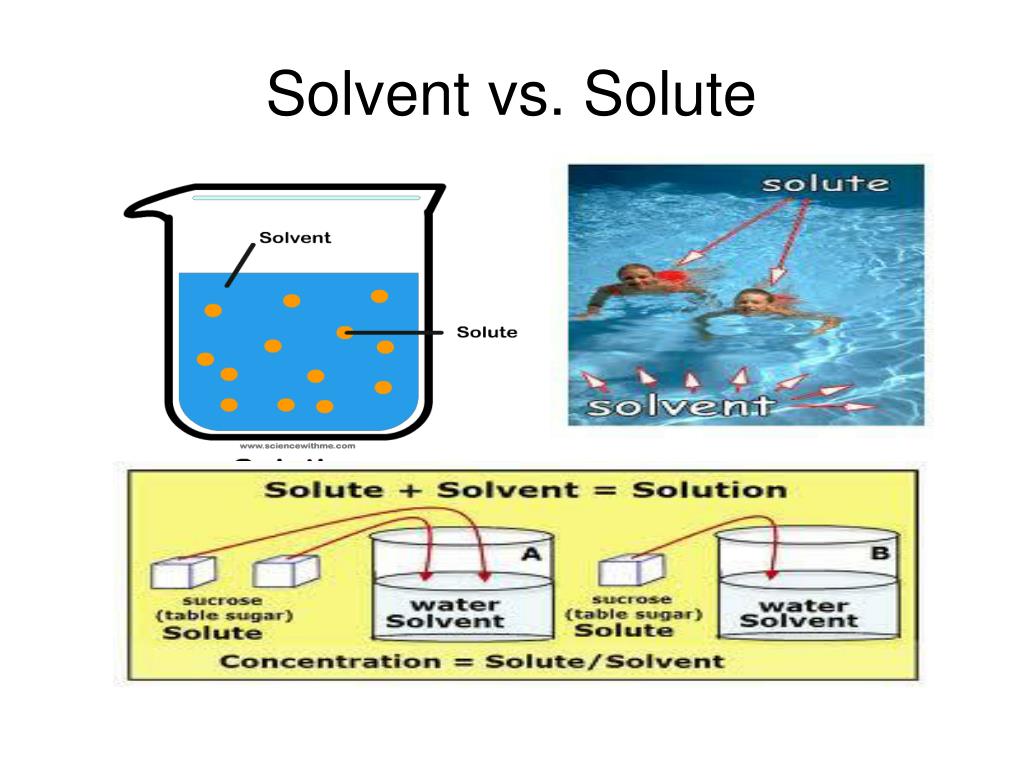
Analyte Vs Solute . If they are not fully interchangeable, then what exactly “analy. So, is there a difference between the terms “analyte” and “analyate”? Tlc is an excellent analytical tool for separating mixtures in a sample. The stationary phase remains fixed in place while the mobile phase carries the components of the mixture through the medium being used. In this section we develop a general theory that we may apply to any form of column chromatography. Partitioning of an analyte between two phases is a thermodynamic process and is characterized by an equilibrium constant, k (function of. Chromatography is a method by which a mixture is separated by distributing its components between two phases. One goal of chromatography is to achieve sharp symmetrical peaks, thus optimizing analyte separation and improving detection. In this section are discussed the details of the separation, and expand upon the general discussion of section 2.1.b. In all forms of chromatography, samples equilibrate between stationary and mobile phases. An analyte, component (in clinical chemistry), titrand (in titrations), or chemical species is a substance or chemical constituent that is of.
from www.slideserve.com
In this section are discussed the details of the separation, and expand upon the general discussion of section 2.1.b. If they are not fully interchangeable, then what exactly “analy. In this section we develop a general theory that we may apply to any form of column chromatography. Partitioning of an analyte between two phases is a thermodynamic process and is characterized by an equilibrium constant, k (function of. So, is there a difference between the terms “analyte” and “analyate”? The stationary phase remains fixed in place while the mobile phase carries the components of the mixture through the medium being used. In all forms of chromatography, samples equilibrate between stationary and mobile phases. Tlc is an excellent analytical tool for separating mixtures in a sample. One goal of chromatography is to achieve sharp symmetrical peaks, thus optimizing analyte separation and improving detection. Chromatography is a method by which a mixture is separated by distributing its components between two phases.
PPT Introduction to Biology BELLWORK PowerPoint Presentation, free
Analyte Vs Solute So, is there a difference between the terms “analyte” and “analyate”? Tlc is an excellent analytical tool for separating mixtures in a sample. In this section we develop a general theory that we may apply to any form of column chromatography. If they are not fully interchangeable, then what exactly “analy. An analyte, component (in clinical chemistry), titrand (in titrations), or chemical species is a substance or chemical constituent that is of. The stationary phase remains fixed in place while the mobile phase carries the components of the mixture through the medium being used. Partitioning of an analyte between two phases is a thermodynamic process and is characterized by an equilibrium constant, k (function of. In this section are discussed the details of the separation, and expand upon the general discussion of section 2.1.b. So, is there a difference between the terms “analyte” and “analyate”? Chromatography is a method by which a mixture is separated by distributing its components between two phases. One goal of chromatography is to achieve sharp symmetrical peaks, thus optimizing analyte separation and improving detection. In all forms of chromatography, samples equilibrate between stationary and mobile phases.
From www.pinterest.com
Solute vs Solvent Biology lessons, Solvent, Science education Analyte Vs Solute Partitioning of an analyte between two phases is a thermodynamic process and is characterized by an equilibrium constant, k (function of. So, is there a difference between the terms “analyte” and “analyate”? An analyte, component (in clinical chemistry), titrand (in titrations), or chemical species is a substance or chemical constituent that is of. Chromatography is a method by which a. Analyte Vs Solute.
From chemistnotes.com
Solute Vs Solvent Definition, Important Characteristics, and Examples Analyte Vs Solute An analyte, component (in clinical chemistry), titrand (in titrations), or chemical species is a substance or chemical constituent that is of. Partitioning of an analyte between two phases is a thermodynamic process and is characterized by an equilibrium constant, k (function of. In this section are discussed the details of the separation, and expand upon the general discussion of section. Analyte Vs Solute.
From www.slideserve.com
PPT Chapter 1516 study guide PowerPoint Presentation, free download Analyte Vs Solute In this section are discussed the details of the separation, and expand upon the general discussion of section 2.1.b. Tlc is an excellent analytical tool for separating mixtures in a sample. One goal of chromatography is to achieve sharp symmetrical peaks, thus optimizing analyte separation and improving detection. In this section we develop a general theory that we may apply. Analyte Vs Solute.
From www.slideserve.com
PPT Solute and Solvent PowerPoint Presentation, free download ID Analyte Vs Solute Tlc is an excellent analytical tool for separating mixtures in a sample. Partitioning of an analyte between two phases is a thermodynamic process and is characterized by an equilibrium constant, k (function of. Chromatography is a method by which a mixture is separated by distributing its components between two phases. In this section are discussed the details of the separation,. Analyte Vs Solute.
From www.difference101.com
Solute vs. Solvent 5 Key Differences, Pros & Cons, Examples Analyte Vs Solute In this section we develop a general theory that we may apply to any form of column chromatography. So, is there a difference between the terms “analyte” and “analyate”? If they are not fully interchangeable, then what exactly “analy. Tlc is an excellent analytical tool for separating mixtures in a sample. Partitioning of an analyte between two phases is a. Analyte Vs Solute.
From thecontentauthority.com
Analyte vs Sample Which Should You Use In Writing? Analyte Vs Solute If they are not fully interchangeable, then what exactly “analy. In all forms of chromatography, samples equilibrate between stationary and mobile phases. In this section are discussed the details of the separation, and expand upon the general discussion of section 2.1.b. Tlc is an excellent analytical tool for separating mixtures in a sample. An analyte, component (in clinical chemistry), titrand. Analyte Vs Solute.
From www.difference101.com
Solute vs. Solvent 5 Key Differences, Pros & Cons, Examples Analyte Vs Solute In this section are discussed the details of the separation, and expand upon the general discussion of section 2.1.b. So, is there a difference between the terms “analyte” and “analyate”? An analyte, component (in clinical chemistry), titrand (in titrations), or chemical species is a substance or chemical constituent that is of. In this section we develop a general theory that. Analyte Vs Solute.
From www.difference101.com
Solute vs. Solvent 5 Key Differences, Pros & Cons, Examples Analyte Vs Solute In all forms of chromatography, samples equilibrate between stationary and mobile phases. The stationary phase remains fixed in place while the mobile phase carries the components of the mixture through the medium being used. Chromatography is a method by which a mixture is separated by distributing its components between two phases. If they are not fully interchangeable, then what exactly. Analyte Vs Solute.
From medium.com
What Is the Difference Between Solute And Solvent? by Diksha Bhardwaj Analyte Vs Solute In this section are discussed the details of the separation, and expand upon the general discussion of section 2.1.b. Tlc is an excellent analytical tool for separating mixtures in a sample. Partitioning of an analyte between two phases is a thermodynamic process and is characterized by an equilibrium constant, k (function of. In all forms of chromatography, samples equilibrate between. Analyte Vs Solute.
From www.difference101.com
Solute vs. Solvent 5 Key Differences, Pros & Cons, Examples Analyte Vs Solute Partitioning of an analyte between two phases is a thermodynamic process and is characterized by an equilibrium constant, k (function of. Tlc is an excellent analytical tool for separating mixtures in a sample. Chromatography is a method by which a mixture is separated by distributing its components between two phases. The stationary phase remains fixed in place while the mobile. Analyte Vs Solute.
From thestudentnotes.com
Solute vs Solvent Definition, 10 Major Differences And Examples The Analyte Vs Solute Chromatography is a method by which a mixture is separated by distributing its components between two phases. The stationary phase remains fixed in place while the mobile phase carries the components of the mixture through the medium being used. So, is there a difference between the terms “analyte” and “analyate”? In this section are discussed the details of the separation,. Analyte Vs Solute.
From arcaneatrium.thebashdogs.com
Soluto y solvente ¡descubre sus diferencias y ejemplos! arcaneatrium Analyte Vs Solute So, is there a difference between the terms “analyte” and “analyate”? An analyte, component (in clinical chemistry), titrand (in titrations), or chemical species is a substance or chemical constituent that is of. Tlc is an excellent analytical tool for separating mixtures in a sample. Partitioning of an analyte between two phases is a thermodynamic process and is characterized by an. Analyte Vs Solute.
From www.aquaportail.com
Analyte définition et explications Analyte Vs Solute One goal of chromatography is to achieve sharp symmetrical peaks, thus optimizing analyte separation and improving detection. In this section are discussed the details of the separation, and expand upon the general discussion of section 2.1.b. In this section we develop a general theory that we may apply to any form of column chromatography. If they are not fully interchangeable,. Analyte Vs Solute.
From jaylin-blogray.blogspot.com
Explain the Difference Between a Solute and a Solvent Analyte Vs Solute The stationary phase remains fixed in place while the mobile phase carries the components of the mixture through the medium being used. If they are not fully interchangeable, then what exactly “analy. In this section we develop a general theory that we may apply to any form of column chromatography. In this section are discussed the details of the separation,. Analyte Vs Solute.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Analyte Vs Solute In all forms of chromatography, samples equilibrate between stationary and mobile phases. Partitioning of an analyte between two phases is a thermodynamic process and is characterized by an equilibrium constant, k (function of. If they are not fully interchangeable, then what exactly “analy. The stationary phase remains fixed in place while the mobile phase carries the components of the mixture. Analyte Vs Solute.
From www.vrogue.co
Solute Vs Solvent Definition 10 Major Differences And vrogue.co Analyte Vs Solute Chromatography is a method by which a mixture is separated by distributing its components between two phases. If they are not fully interchangeable, then what exactly “analy. Partitioning of an analyte between two phases is a thermodynamic process and is characterized by an equilibrium constant, k (function of. Tlc is an excellent analytical tool for separating mixtures in a sample.. Analyte Vs Solute.
From www.differencebetween.com
Difference Between Solvent and Solute Compare the Difference Between Analyte Vs Solute In this section are discussed the details of the separation, and expand upon the general discussion of section 2.1.b. An analyte, component (in clinical chemistry), titrand (in titrations), or chemical species is a substance or chemical constituent that is of. So, is there a difference between the terms “analyte” and “analyate”? In all forms of chromatography, samples equilibrate between stationary. Analyte Vs Solute.
From testbook.com
Solute Vs Solvent Learn its Definition ,Types & their Examples Analyte Vs Solute In this section are discussed the details of the separation, and expand upon the general discussion of section 2.1.b. If they are not fully interchangeable, then what exactly “analy. The stationary phase remains fixed in place while the mobile phase carries the components of the mixture through the medium being used. One goal of chromatography is to achieve sharp symmetrical. Analyte Vs Solute.
From slideplayer.com
Chem. 231 2/25 Lecture. ppt download Analyte Vs Solute Chromatography is a method by which a mixture is separated by distributing its components between two phases. In all forms of chromatography, samples equilibrate between stationary and mobile phases. So, is there a difference between the terms “analyte” and “analyate”? If they are not fully interchangeable, then what exactly “analy. An analyte, component (in clinical chemistry), titrand (in titrations), or. Analyte Vs Solute.
From www.slideserve.com
PPT Introduction to Biology BELLWORK PowerPoint Presentation, free Analyte Vs Solute Partitioning of an analyte between two phases is a thermodynamic process and is characterized by an equilibrium constant, k (function of. Chromatography is a method by which a mixture is separated by distributing its components between two phases. The stationary phase remains fixed in place while the mobile phase carries the components of the mixture through the medium being used.. Analyte Vs Solute.
From www.youtube.com
Difference between solute and solvent YouTube Analyte Vs Solute One goal of chromatography is to achieve sharp symmetrical peaks, thus optimizing analyte separation and improving detection. So, is there a difference between the terms “analyte” and “analyate”? Partitioning of an analyte between two phases is a thermodynamic process and is characterized by an equilibrium constant, k (function of. The stationary phase remains fixed in place while the mobile phase. Analyte Vs Solute.
From www.pinterest.com
titration problems Teaching chemistry, Chemistry lessons, High school Analyte Vs Solute In all forms of chromatography, samples equilibrate between stationary and mobile phases. So, is there a difference between the terms “analyte” and “analyate”? In this section are discussed the details of the separation, and expand upon the general discussion of section 2.1.b. One goal of chromatography is to achieve sharp symmetrical peaks, thus optimizing analyte separation and improving detection. The. Analyte Vs Solute.
From lessonlibraryemersed.z13.web.core.windows.net
Titration Practical Questions And Answers Analyte Vs Solute Partitioning of an analyte between two phases is a thermodynamic process and is characterized by an equilibrium constant, k (function of. In all forms of chromatography, samples equilibrate between stationary and mobile phases. The stationary phase remains fixed in place while the mobile phase carries the components of the mixture through the medium being used. If they are not fully. Analyte Vs Solute.
From ar.inspiredpencil.com
Solvent And Solute Analyte Vs Solute So, is there a difference between the terms “analyte” and “analyate”? An analyte, component (in clinical chemistry), titrand (in titrations), or chemical species is a substance or chemical constituent that is of. In this section are discussed the details of the separation, and expand upon the general discussion of section 2.1.b. The stationary phase remains fixed in place while the. Analyte Vs Solute.
From pediaa.com
Difference Between Solvent and Solute Definition, Properties, Examples Analyte Vs Solute In all forms of chromatography, samples equilibrate between stationary and mobile phases. So, is there a difference between the terms “analyte” and “analyate”? If they are not fully interchangeable, then what exactly “analy. One goal of chromatography is to achieve sharp symmetrical peaks, thus optimizing analyte separation and improving detection. An analyte, component (in clinical chemistry), titrand (in titrations), or. Analyte Vs Solute.
From www.difference101.com
Solute vs. Solvent 5 Key Differences, Pros & Cons, Examples Analyte Vs Solute In all forms of chromatography, samples equilibrate between stationary and mobile phases. So, is there a difference between the terms “analyte” and “analyate”? Tlc is an excellent analytical tool for separating mixtures in a sample. The stationary phase remains fixed in place while the mobile phase carries the components of the mixture through the medium being used. In this section. Analyte Vs Solute.
From www.youtube.com
Solution with examples Identify the solute and solvent YouTube Analyte Vs Solute In all forms of chromatography, samples equilibrate between stationary and mobile phases. In this section we develop a general theory that we may apply to any form of column chromatography. The stationary phase remains fixed in place while the mobile phase carries the components of the mixture through the medium being used. If they are not fully interchangeable, then what. Analyte Vs Solute.
From chemistnotes.com
Solute Vs Solvent Definition, Important Characteristics, and Examples Analyte Vs Solute Tlc is an excellent analytical tool for separating mixtures in a sample. Partitioning of an analyte between two phases is a thermodynamic process and is characterized by an equilibrium constant, k (function of. An analyte, component (in clinical chemistry), titrand (in titrations), or chemical species is a substance or chemical constituent that is of. Chromatography is a method by which. Analyte Vs Solute.
From thecontentauthority.com
Analysis vs Analyte When To Use Each One? What To Consider Analyte Vs Solute In this section we develop a general theory that we may apply to any form of column chromatography. If they are not fully interchangeable, then what exactly “analy. Chromatography is a method by which a mixture is separated by distributing its components between two phases. An analyte, component (in clinical chemistry), titrand (in titrations), or chemical species is a substance. Analyte Vs Solute.
From general.chemistrysteps.com
AcidBase Titrations Chemistry Steps Analyte Vs Solute If they are not fully interchangeable, then what exactly “analy. Tlc is an excellent analytical tool for separating mixtures in a sample. Partitioning of an analyte between two phases is a thermodynamic process and is characterized by an equilibrium constant, k (function of. An analyte, component (in clinical chemistry), titrand (in titrations), or chemical species is a substance or chemical. Analyte Vs Solute.
From www.slideserve.com
PPT Solutions PowerPoint Presentation, free download ID2175158 Analyte Vs Solute Partitioning of an analyte between two phases is a thermodynamic process and is characterized by an equilibrium constant, k (function of. If they are not fully interchangeable, then what exactly “analy. One goal of chromatography is to achieve sharp symmetrical peaks, thus optimizing analyte separation and improving detection. An analyte, component (in clinical chemistry), titrand (in titrations), or chemical species. Analyte Vs Solute.
From www.youtube.com
solute and solvent difference Difference between solute and solvent Analyte Vs Solute In this section are discussed the details of the separation, and expand upon the general discussion of section 2.1.b. In all forms of chromatography, samples equilibrate between stationary and mobile phases. The stationary phase remains fixed in place while the mobile phase carries the components of the mixture through the medium being used. An analyte, component (in clinical chemistry), titrand. Analyte Vs Solute.
From www.vrogue.co
Solute Vs Solvent Definition 10 Major Differences And vrogue.co Analyte Vs Solute Chromatography is a method by which a mixture is separated by distributing its components between two phases. So, is there a difference between the terms “analyte” and “analyate”? Partitioning of an analyte between two phases is a thermodynamic process and is characterized by an equilibrium constant, k (function of. The stationary phase remains fixed in place while the mobile phase. Analyte Vs Solute.
From www.difference101.com
Solute vs. Solvent 5 Key Differences, Pros & Cons, Examples Analyte Vs Solute So, is there a difference between the terms “analyte” and “analyate”? In all forms of chromatography, samples equilibrate between stationary and mobile phases. One goal of chromatography is to achieve sharp symmetrical peaks, thus optimizing analyte separation and improving detection. Chromatography is a method by which a mixture is separated by distributing its components between two phases. In this section. Analyte Vs Solute.
From askanydifference.com
Solvent vs Solute Difference and Comparison Analyte Vs Solute In this section are discussed the details of the separation, and expand upon the general discussion of section 2.1.b. Chromatography is a method by which a mixture is separated by distributing its components between two phases. The stationary phase remains fixed in place while the mobile phase carries the components of the mixture through the medium being used. If they. Analyte Vs Solute.