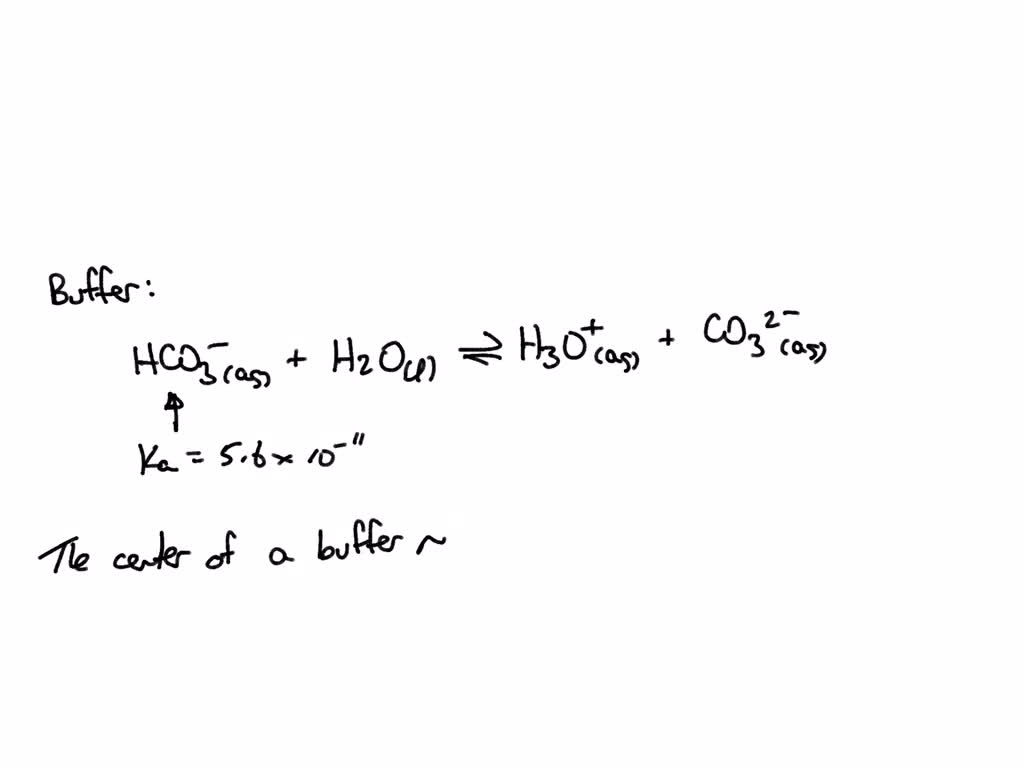How Successful Was The Laboratory Buffer In Resisting Changes In Ph . A molecule or molecular system that acts to resist changes in ph. Buffers contain a weak acid ( ha ) and its conjugate weak base. The strong acid reacts with the weak base in the buffer to form a weak acid, which produces few h ions in solution and. How does a buffer resist change in ph upon addition of a strong acid? This mechanism involves a buffer, a solution that resists dramatic changes in ph. Buffers are solutions that resist a change in ph after adding an acid or a base. Buffers do so by being composed of certain. The ability of a buffer to resist changes in ph is colorfully shown by adding strong acid and base to water and buffer. How successful was the buffer in resisting changes in p h when the additional drops of the aci originals An aqueous buffer has the ability to resist, for a time, changes in ph that are caused by the addition of strong acids or strong bases.
from www.numerade.com
How does a buffer resist change in ph upon addition of a strong acid? A molecule or molecular system that acts to resist changes in ph. The strong acid reacts with the weak base in the buffer to form a weak acid, which produces few h ions in solution and. Buffers are solutions that resist a change in ph after adding an acid or a base. This mechanism involves a buffer, a solution that resists dramatic changes in ph. The ability of a buffer to resist changes in ph is colorfully shown by adding strong acid and base to water and buffer. Buffers contain a weak acid ( ha ) and its conjugate weak base. Buffers do so by being composed of certain. How successful was the buffer in resisting changes in p h when the additional drops of the aci originals An aqueous buffer has the ability to resist, for a time, changes in ph that are caused by the addition of strong acids or strong bases.
SOLVED What is the pH at which a buffer composed of CO32 and HCO3
How Successful Was The Laboratory Buffer In Resisting Changes In Ph Buffers are solutions that resist a change in ph after adding an acid or a base. Buffers do so by being composed of certain. An aqueous buffer has the ability to resist, for a time, changes in ph that are caused by the addition of strong acids or strong bases. Buffers are solutions that resist a change in ph after adding an acid or a base. The ability of a buffer to resist changes in ph is colorfully shown by adding strong acid and base to water and buffer. A molecule or molecular system that acts to resist changes in ph. This mechanism involves a buffer, a solution that resists dramatic changes in ph. The strong acid reacts with the weak base in the buffer to form a weak acid, which produces few h ions in solution and. How does a buffer resist change in ph upon addition of a strong acid? How successful was the buffer in resisting changes in p h when the additional drops of the aci originals Buffers contain a weak acid ( ha ) and its conjugate weak base.
From www.studocu.com
Introduction to Buffers INTRODUCTION TO BUFFERS A buffer is a How Successful Was The Laboratory Buffer In Resisting Changes In Ph The strong acid reacts with the weak base in the buffer to form a weak acid, which produces few h ions in solution and. How successful was the buffer in resisting changes in p h when the additional drops of the aci originals The ability of a buffer to resist changes in ph is colorfully shown by adding strong acid. How Successful Was The Laboratory Buffer In Resisting Changes In Ph.
From www.coursehero.com
[Solved] A buffer is a solution that has the ability to resist changes How Successful Was The Laboratory Buffer In Resisting Changes In Ph The strong acid reacts with the weak base in the buffer to form a weak acid, which produces few h ions in solution and. Buffers are solutions that resist a change in ph after adding an acid or a base. Buffers contain a weak acid ( ha ) and its conjugate weak base. A molecule or molecular system that acts. How Successful Was The Laboratory Buffer In Resisting Changes In Ph.
From psiberg.com
Buffer Solutions Principle and Mechanism of their Action PSIBERG How Successful Was The Laboratory Buffer In Resisting Changes In Ph The strong acid reacts with the weak base in the buffer to form a weak acid, which produces few h ions in solution and. Buffers are solutions that resist a change in ph after adding an acid or a base. How does a buffer resist change in ph upon addition of a strong acid? The ability of a buffer to. How Successful Was The Laboratory Buffer In Resisting Changes In Ph.
From slideplayer.com
Chapter 17 buffers resist changes in pH by neutralizing added acid or How Successful Was The Laboratory Buffer In Resisting Changes In Ph An aqueous buffer has the ability to resist, for a time, changes in ph that are caused by the addition of strong acids or strong bases. How successful was the buffer in resisting changes in p h when the additional drops of the aci originals This mechanism involves a buffer, a solution that resists dramatic changes in ph. Buffers contain. How Successful Was The Laboratory Buffer In Resisting Changes In Ph.
From slideplayer.com
Chapter 17 buffers resist changes in pH by neutralizing added acid or How Successful Was The Laboratory Buffer In Resisting Changes In Ph How successful was the buffer in resisting changes in p h when the additional drops of the aci originals Buffers do so by being composed of certain. The ability of a buffer to resist changes in ph is colorfully shown by adding strong acid and base to water and buffer. The strong acid reacts with the weak base in the. How Successful Was The Laboratory Buffer In Resisting Changes In Ph.
From www.numerade.com
SOLVED What is the pH at which a buffer composed of CH3NH2 and CH3NH3 How Successful Was The Laboratory Buffer In Resisting Changes In Ph This mechanism involves a buffer, a solution that resists dramatic changes in ph. A molecule or molecular system that acts to resist changes in ph. An aqueous buffer has the ability to resist, for a time, changes in ph that are caused by the addition of strong acids or strong bases. Buffers are solutions that resist a change in ph. How Successful Was The Laboratory Buffer In Resisting Changes In Ph.
From slideplayer.com
Introduction into biochemistry ppt download How Successful Was The Laboratory Buffer In Resisting Changes In Ph Buffers do so by being composed of certain. This mechanism involves a buffer, a solution that resists dramatic changes in ph. The strong acid reacts with the weak base in the buffer to form a weak acid, which produces few h ions in solution and. How does a buffer resist change in ph upon addition of a strong acid? How. How Successful Was The Laboratory Buffer In Resisting Changes In Ph.
From slideplayer.com
Chapter 17 buffers resist changes in pH by neutralizing added acid or How Successful Was The Laboratory Buffer In Resisting Changes In Ph Buffers are solutions that resist a change in ph after adding an acid or a base. The strong acid reacts with the weak base in the buffer to form a weak acid, which produces few h ions in solution and. Buffers contain a weak acid ( ha ) and its conjugate weak base. How successful was the buffer in resisting. How Successful Was The Laboratory Buffer In Resisting Changes In Ph.
From www.chegg.com
Solved Introduction The buffer effect resisting the pH How Successful Was The Laboratory Buffer In Resisting Changes In Ph The ability of a buffer to resist changes in ph is colorfully shown by adding strong acid and base to water and buffer. Buffers do so by being composed of certain. Buffers are solutions that resist a change in ph after adding an acid or a base. How does a buffer resist change in ph upon addition of a strong. How Successful Was The Laboratory Buffer In Resisting Changes In Ph.
From www.youtube.com
How buffer solution resist change in PH ??? YouTube How Successful Was The Laboratory Buffer In Resisting Changes In Ph An aqueous buffer has the ability to resist, for a time, changes in ph that are caused by the addition of strong acids or strong bases. The strong acid reacts with the weak base in the buffer to form a weak acid, which produces few h ions in solution and. A molecule or molecular system that acts to resist changes. How Successful Was The Laboratory Buffer In Resisting Changes In Ph.
From www.coursehero.com
[Solved] A buffer is a solution that has the ability to resist changes How Successful Was The Laboratory Buffer In Resisting Changes In Ph The ability of a buffer to resist changes in ph is colorfully shown by adding strong acid and base to water and buffer. An aqueous buffer has the ability to resist, for a time, changes in ph that are caused by the addition of strong acids or strong bases. Buffers contain a weak acid ( ha ) and its conjugate. How Successful Was The Laboratory Buffer In Resisting Changes In Ph.
From www.numerade.com
SOLVED What is the pH at which a buffer composed of CO32 and HCO3 How Successful Was The Laboratory Buffer In Resisting Changes In Ph How does a buffer resist change in ph upon addition of a strong acid? This mechanism involves a buffer, a solution that resists dramatic changes in ph. Buffers are solutions that resist a change in ph after adding an acid or a base. The strong acid reacts with the weak base in the buffer to form a weak acid, which. How Successful Was The Laboratory Buffer In Resisting Changes In Ph.
From slideplayer.com
Buffers weak acids or bases that resist pH changes ppt download How Successful Was The Laboratory Buffer In Resisting Changes In Ph An aqueous buffer has the ability to resist, for a time, changes in ph that are caused by the addition of strong acids or strong bases. Buffers do so by being composed of certain. The ability of a buffer to resist changes in ph is colorfully shown by adding strong acid and base to water and buffer. How does a. How Successful Was The Laboratory Buffer In Resisting Changes In Ph.
From www.youtube.com
14.10 Buffers Solutions that Resist pH Change YouTube How Successful Was The Laboratory Buffer In Resisting Changes In Ph The strong acid reacts with the weak base in the buffer to form a weak acid, which produces few h ions in solution and. Buffers are solutions that resist a change in ph after adding an acid or a base. Buffers contain a weak acid ( ha ) and its conjugate weak base. This mechanism involves a buffer, a solution. How Successful Was The Laboratory Buffer In Resisting Changes In Ph.
From www.numerade.com
SOLVED What is the pH at which a buffer composed of HOCl and OCl How Successful Was The Laboratory Buffer In Resisting Changes In Ph This mechanism involves a buffer, a solution that resists dramatic changes in ph. An aqueous buffer has the ability to resist, for a time, changes in ph that are caused by the addition of strong acids or strong bases. How successful was the buffer in resisting changes in p h when the additional drops of the aci originals Buffers contain. How Successful Was The Laboratory Buffer In Resisting Changes In Ph.
From www.youtube.com
Chapter 14d how do buffers resist pH changes YouTube How Successful Was The Laboratory Buffer In Resisting Changes In Ph A molecule or molecular system that acts to resist changes in ph. Buffers contain a weak acid ( ha ) and its conjugate weak base. Buffers do so by being composed of certain. How does a buffer resist change in ph upon addition of a strong acid? The ability of a buffer to resist changes in ph is colorfully shown. How Successful Was The Laboratory Buffer In Resisting Changes In Ph.
From slideplayer.com
Buffers A buffer is a mixture of chemicals that make a solution resist How Successful Was The Laboratory Buffer In Resisting Changes In Ph Buffers do so by being composed of certain. An aqueous buffer has the ability to resist, for a time, changes in ph that are caused by the addition of strong acids or strong bases. A molecule or molecular system that acts to resist changes in ph. The ability of a buffer to resist changes in ph is colorfully shown by. How Successful Was The Laboratory Buffer In Resisting Changes In Ph.
From chem.libretexts.org
8.8 Buffers Solutions That Resist pH Change Chemistry LibreTexts How Successful Was The Laboratory Buffer In Resisting Changes In Ph Buffers do so by being composed of certain. The ability of a buffer to resist changes in ph is colorfully shown by adding strong acid and base to water and buffer. A molecule or molecular system that acts to resist changes in ph. The strong acid reacts with the weak base in the buffer to form a weak acid, which. How Successful Was The Laboratory Buffer In Resisting Changes In Ph.
From www.slideserve.com
PPT Buffer solutions PowerPoint Presentation, free download ID6578454 How Successful Was The Laboratory Buffer In Resisting Changes In Ph The ability of a buffer to resist changes in ph is colorfully shown by adding strong acid and base to water and buffer. Buffers do so by being composed of certain. This mechanism involves a buffer, a solution that resists dramatic changes in ph. Buffers contain a weak acid ( ha ) and its conjugate weak base. Buffers are solutions. How Successful Was The Laboratory Buffer In Resisting Changes In Ph.
From www.quanswer.com
How do buffers work to resist changes in pH? Quanswer How Successful Was The Laboratory Buffer In Resisting Changes In Ph An aqueous buffer has the ability to resist, for a time, changes in ph that are caused by the addition of strong acids or strong bases. Buffers contain a weak acid ( ha ) and its conjugate weak base. Buffers are solutions that resist a change in ph after adding an acid or a base. A molecule or molecular system. How Successful Was The Laboratory Buffer In Resisting Changes In Ph.
From www.brainkart.com
Buffers How Successful Was The Laboratory Buffer In Resisting Changes In Ph How successful was the buffer in resisting changes in p h when the additional drops of the aci originals Buffers are solutions that resist a change in ph after adding an acid or a base. This mechanism involves a buffer, a solution that resists dramatic changes in ph. The ability of a buffer to resist changes in ph is colorfully. How Successful Was The Laboratory Buffer In Resisting Changes In Ph.
From www.chegg.com
Solved Introduction The buffer effect resisting the pH How Successful Was The Laboratory Buffer In Resisting Changes In Ph This mechanism involves a buffer, a solution that resists dramatic changes in ph. Buffers are solutions that resist a change in ph after adding an acid or a base. How successful was the buffer in resisting changes in p h when the additional drops of the aci originals A molecule or molecular system that acts to resist changes in ph.. How Successful Was The Laboratory Buffer In Resisting Changes In Ph.
From derangedphysiology.com
Buffers and buffering power Deranged Physiology How Successful Was The Laboratory Buffer In Resisting Changes In Ph How does a buffer resist change in ph upon addition of a strong acid? The strong acid reacts with the weak base in the buffer to form a weak acid, which produces few h ions in solution and. The ability of a buffer to resist changes in ph is colorfully shown by adding strong acid and base to water and. How Successful Was The Laboratory Buffer In Resisting Changes In Ph.
From www.youtube.com
Buffer Action Acidic and basic buffer Action how buffer resist pH How Successful Was The Laboratory Buffer In Resisting Changes In Ph How successful was the buffer in resisting changes in p h when the additional drops of the aci originals Buffers do so by being composed of certain. The ability of a buffer to resist changes in ph is colorfully shown by adding strong acid and base to water and buffer. The strong acid reacts with the weak base in the. How Successful Was The Laboratory Buffer In Resisting Changes In Ph.
From www.bartleby.com
Answered At which corresponding pH value(s) is a… bartleby How Successful Was The Laboratory Buffer In Resisting Changes In Ph How does a buffer resist change in ph upon addition of a strong acid? A molecule or molecular system that acts to resist changes in ph. How successful was the buffer in resisting changes in p h when the additional drops of the aci originals Buffers contain a weak acid ( ha ) and its conjugate weak base. Buffers are. How Successful Was The Laboratory Buffer In Resisting Changes In Ph.
From www.slideserve.com
PPT Section 2 Chemical and Biologic Foundations of Biochemistry How Successful Was The Laboratory Buffer In Resisting Changes In Ph This mechanism involves a buffer, a solution that resists dramatic changes in ph. The strong acid reacts with the weak base in the buffer to form a weak acid, which produces few h ions in solution and. The ability of a buffer to resist changes in ph is colorfully shown by adding strong acid and base to water and buffer.. How Successful Was The Laboratory Buffer In Resisting Changes In Ph.
From www.coursehero.com
[Solved] A buffer is a solution that has the ability to resist changes How Successful Was The Laboratory Buffer In Resisting Changes In Ph This mechanism involves a buffer, a solution that resists dramatic changes in ph. Buffers are solutions that resist a change in ph after adding an acid or a base. Buffers contain a weak acid ( ha ) and its conjugate weak base. A molecule or molecular system that acts to resist changes in ph. How successful was the buffer in. How Successful Was The Laboratory Buffer In Resisting Changes In Ph.
From www.numerade.com
SOLVED How successful was the Laboratory Buffer (pH T) in resisting How Successful Was The Laboratory Buffer In Resisting Changes In Ph How successful was the buffer in resisting changes in p h when the additional drops of the aci originals Buffers are solutions that resist a change in ph after adding an acid or a base. An aqueous buffer has the ability to resist, for a time, changes in ph that are caused by the addition of strong acids or strong. How Successful Was The Laboratory Buffer In Resisting Changes In Ph.
From www.numerade.com
SOLVED Categorize each statement as true or false Buffers are How Successful Was The Laboratory Buffer In Resisting Changes In Ph An aqueous buffer has the ability to resist, for a time, changes in ph that are caused by the addition of strong acids or strong bases. This mechanism involves a buffer, a solution that resists dramatic changes in ph. Buffers do so by being composed of certain. How successful was the buffer in resisting changes in p h when the. How Successful Was The Laboratory Buffer In Resisting Changes In Ph.
From www.chegg.com
Solved a. Buffers resist the change in pH when small amounts How Successful Was The Laboratory Buffer In Resisting Changes In Ph Buffers contain a weak acid ( ha ) and its conjugate weak base. How successful was the buffer in resisting changes in p h when the additional drops of the aci originals The ability of a buffer to resist changes in ph is colorfully shown by adding strong acid and base to water and buffer. A molecule or molecular system. How Successful Was The Laboratory Buffer In Resisting Changes In Ph.
From www.slideserve.com
PPT THEME Acidbase equilibrium in biological systems. Buffer How Successful Was The Laboratory Buffer In Resisting Changes In Ph Buffers contain a weak acid ( ha ) and its conjugate weak base. How successful was the buffer in resisting changes in p h when the additional drops of the aci originals Buffers are solutions that resist a change in ph after adding an acid or a base. This mechanism involves a buffer, a solution that resists dramatic changes in. How Successful Was The Laboratory Buffer In Resisting Changes In Ph.
From www.numerade.com
SOLVED Categorize each statement as true or false Buffers are How Successful Was The Laboratory Buffer In Resisting Changes In Ph The strong acid reacts with the weak base in the buffer to form a weak acid, which produces few h ions in solution and. A molecule or molecular system that acts to resist changes in ph. How successful was the buffer in resisting changes in p h when the additional drops of the aci originals How does a buffer resist. How Successful Was The Laboratory Buffer In Resisting Changes In Ph.
From www.youtube.com
20 14.10 Buffers Solutions That Resist pH Change YouTube How Successful Was The Laboratory Buffer In Resisting Changes In Ph Buffers are solutions that resist a change in ph after adding an acid or a base. The ability of a buffer to resist changes in ph is colorfully shown by adding strong acid and base to water and buffer. How does a buffer resist change in ph upon addition of a strong acid? Buffers contain a weak acid ( ha. How Successful Was The Laboratory Buffer In Resisting Changes In Ph.
From www.slideserve.com
PPT pH and Buffers PowerPoint Presentation, free download ID2948821 How Successful Was The Laboratory Buffer In Resisting Changes In Ph Buffers are solutions that resist a change in ph after adding an acid or a base. The ability of a buffer to resist changes in ph is colorfully shown by adding strong acid and base to water and buffer. The strong acid reacts with the weak base in the buffer to form a weak acid, which produces few h ions. How Successful Was The Laboratory Buffer In Resisting Changes In Ph.
From www.slideserve.com
PPT Chapter 19 Reactions of Acids and Bases PowerPoint Presentation How Successful Was The Laboratory Buffer In Resisting Changes In Ph An aqueous buffer has the ability to resist, for a time, changes in ph that are caused by the addition of strong acids or strong bases. This mechanism involves a buffer, a solution that resists dramatic changes in ph. How successful was the buffer in resisting changes in p h when the additional drops of the aci originals Buffers do. How Successful Was The Laboratory Buffer In Resisting Changes In Ph.