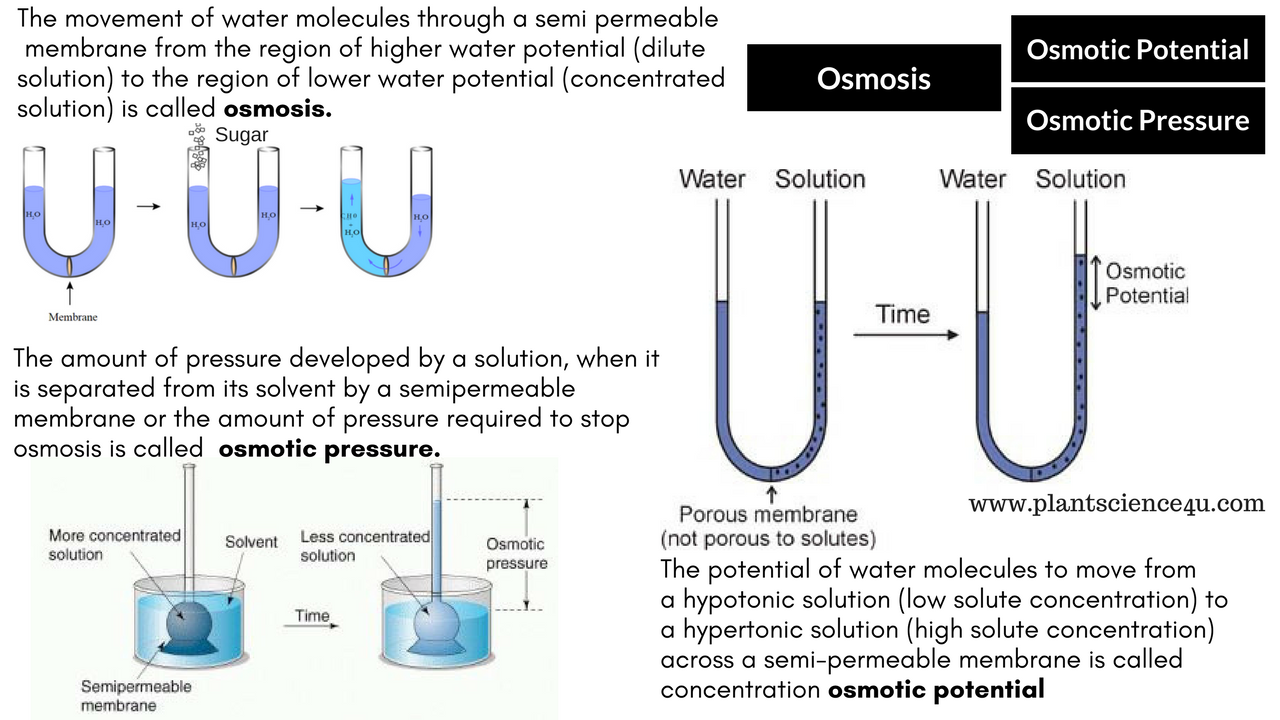
Osmometer Pressure Definition . In other words, it is the pressure of a solvent against a semipermeable membrane that seeks to equalize the concentration of a solution on both sides of the membrane. Osmotic pressure is the minimum pressure that prevents solvent molecules (water) from flowing through a semipermeable membrane. In essence, the osmotic pressure is the pressure needed to stop the flow of water or osmosis. Osmotic pressure is the pressure caused by a difference in the amounts of solutes (or molecules) between solutions (or fluids) separated by a semipermeable membrane. Osmotic pressure is the pressure a solvent exerts to prevent the inward flow of solvent molecules across a semipermeable membrane due to a difference in solute concentration on either side of the membrane. Osmotic pressure can be thought of as the pressure that would be required to stop water from diffusing through a barrier by. Osmotic pressure is the pressure required to stop the net movement of water across a permeable membrane that divides the solvent and solution, whereas oncotic pressure is the contribution of colloids to total osmolality.
from www.plantscience4u.com
Osmotic pressure is the pressure caused by a difference in the amounts of solutes (or molecules) between solutions (or fluids) separated by a semipermeable membrane. Osmotic pressure is the pressure a solvent exerts to prevent the inward flow of solvent molecules across a semipermeable membrane due to a difference in solute concentration on either side of the membrane. Osmotic pressure is the minimum pressure that prevents solvent molecules (water) from flowing through a semipermeable membrane. Osmotic pressure is the pressure required to stop the net movement of water across a permeable membrane that divides the solvent and solution, whereas oncotic pressure is the contribution of colloids to total osmolality. In other words, it is the pressure of a solvent against a semipermeable membrane that seeks to equalize the concentration of a solution on both sides of the membrane. In essence, the osmotic pressure is the pressure needed to stop the flow of water or osmosis. Osmotic pressure can be thought of as the pressure that would be required to stop water from diffusing through a barrier by.
Difference between Osmotic pressure and Osmotic potential
Osmometer Pressure Definition Osmotic pressure can be thought of as the pressure that would be required to stop water from diffusing through a barrier by. Osmotic pressure can be thought of as the pressure that would be required to stop water from diffusing through a barrier by. In essence, the osmotic pressure is the pressure needed to stop the flow of water or osmosis. Osmotic pressure is the minimum pressure that prevents solvent molecules (water) from flowing through a semipermeable membrane. Osmotic pressure is the pressure required to stop the net movement of water across a permeable membrane that divides the solvent and solution, whereas oncotic pressure is the contribution of colloids to total osmolality. In other words, it is the pressure of a solvent against a semipermeable membrane that seeks to equalize the concentration of a solution on both sides of the membrane. Osmotic pressure is the pressure caused by a difference in the amounts of solutes (or molecules) between solutions (or fluids) separated by a semipermeable membrane. Osmotic pressure is the pressure a solvent exerts to prevent the inward flow of solvent molecules across a semipermeable membrane due to a difference in solute concentration on either side of the membrane.
From www.scribd.com
Osmotic Pressure Definition, Formula, Examples, Solved Exercises Osmometer Pressure Definition Osmotic pressure can be thought of as the pressure that would be required to stop water from diffusing through a barrier by. In essence, the osmotic pressure is the pressure needed to stop the flow of water or osmosis. Osmotic pressure is the pressure a solvent exerts to prevent the inward flow of solvent molecules across a semipermeable membrane due. Osmometer Pressure Definition.
From www.biologyonline.com
Osmotic pressure Definition and Examples Biology Online Dictionary Osmometer Pressure Definition Osmotic pressure is the minimum pressure that prevents solvent molecules (water) from flowing through a semipermeable membrane. Osmotic pressure is the pressure caused by a difference in the amounts of solutes (or molecules) between solutions (or fluids) separated by a semipermeable membrane. In other words, it is the pressure of a solvent against a semipermeable membrane that seeks to equalize. Osmometer Pressure Definition.
From pediaa.com
Difference Between Osmotic Pressure and Oncotic Pressure Osmometer Pressure Definition Osmotic pressure is the pressure required to stop the net movement of water across a permeable membrane that divides the solvent and solution, whereas oncotic pressure is the contribution of colloids to total osmolality. In other words, it is the pressure of a solvent against a semipermeable membrane that seeks to equalize the concentration of a solution on both sides. Osmometer Pressure Definition.
From www.examples.com
Osmosis Definition, Osmotic Solutions, Types, Effect of Osmosis on Osmometer Pressure Definition In other words, it is the pressure of a solvent against a semipermeable membrane that seeks to equalize the concentration of a solution on both sides of the membrane. Osmotic pressure is the pressure a solvent exerts to prevent the inward flow of solvent molecules across a semipermeable membrane due to a difference in solute concentration on either side of. Osmometer Pressure Definition.
From www.youtube.com
Osmotic Pressure Osmosis Colligative property Physiology Series Osmometer Pressure Definition In essence, the osmotic pressure is the pressure needed to stop the flow of water or osmosis. Osmotic pressure can be thought of as the pressure that would be required to stop water from diffusing through a barrier by. Osmotic pressure is the pressure required to stop the net movement of water across a permeable membrane that divides the solvent. Osmometer Pressure Definition.
From thechemistrynotes.com
Osmotic Pressure Definition, Formulas, Laws Osmometer Pressure Definition Osmotic pressure can be thought of as the pressure that would be required to stop water from diffusing through a barrier by. Osmotic pressure is the pressure required to stop the net movement of water across a permeable membrane that divides the solvent and solution, whereas oncotic pressure is the contribution of colloids to total osmolality. Osmotic pressure is the. Osmometer Pressure Definition.
From www.plantscience4u.com
Difference between Osmotic pressure and Osmotic potential Osmometer Pressure Definition Osmotic pressure is the minimum pressure that prevents solvent molecules (water) from flowing through a semipermeable membrane. Osmotic pressure is the pressure required to stop the net movement of water across a permeable membrane that divides the solvent and solution, whereas oncotic pressure is the contribution of colloids to total osmolality. Osmotic pressure is the pressure a solvent exerts to. Osmometer Pressure Definition.
From www.priyamstudycentre.com
Osmosis Definition, Example, Osmotic Pressure Osmometer Pressure Definition Osmotic pressure is the pressure caused by a difference in the amounts of solutes (or molecules) between solutions (or fluids) separated by a semipermeable membrane. Osmotic pressure is the pressure required to stop the net movement of water across a permeable membrane that divides the solvent and solution, whereas oncotic pressure is the contribution of colloids to total osmolality. In. Osmometer Pressure Definition.
From www.javatpoint.com
Osmotic Pressure Definition JavaTpoint Osmometer Pressure Definition Osmotic pressure is the pressure caused by a difference in the amounts of solutes (or molecules) between solutions (or fluids) separated by a semipermeable membrane. Osmotic pressure can be thought of as the pressure that would be required to stop water from diffusing through a barrier by. Osmotic pressure is the minimum pressure that prevents solvent molecules (water) from flowing. Osmometer Pressure Definition.
From scienceinfo.com
Osmotic Pressure Definition, Formulas, Laws Osmometer Pressure Definition In other words, it is the pressure of a solvent against a semipermeable membrane that seeks to equalize the concentration of a solution on both sides of the membrane. Osmotic pressure is the pressure required to stop the net movement of water across a permeable membrane that divides the solvent and solution, whereas oncotic pressure is the contribution of colloids. Osmometer Pressure Definition.
From gbu-presnenskij.ru
Osmotic Pressure Definition, Formula, Examples, 47 OFF Osmometer Pressure Definition Osmotic pressure is the pressure required to stop the net movement of water across a permeable membrane that divides the solvent and solution, whereas oncotic pressure is the contribution of colloids to total osmolality. Osmotic pressure can be thought of as the pressure that would be required to stop water from diffusing through a barrier by. In other words, it. Osmometer Pressure Definition.
From preparatorychemistry.com
Osmosis Osmometer Pressure Definition Osmotic pressure is the pressure a solvent exerts to prevent the inward flow of solvent molecules across a semipermeable membrane due to a difference in solute concentration on either side of the membrane. In other words, it is the pressure of a solvent against a semipermeable membrane that seeks to equalize the concentration of a solution on both sides of. Osmometer Pressure Definition.
From dauglas.afphila.com
Osmosis Definition, Osmotic Pressure, Formula, Examples, & FAQs Osmometer Pressure Definition Osmotic pressure is the pressure a solvent exerts to prevent the inward flow of solvent molecules across a semipermeable membrane due to a difference in solute concentration on either side of the membrane. Osmotic pressure is the minimum pressure that prevents solvent molecules (water) from flowing through a semipermeable membrane. In other words, it is the pressure of a solvent. Osmometer Pressure Definition.
From www.chemistrylearner.com
Osmotic Pressure Definition, Formula, and Examples Osmometer Pressure Definition Osmotic pressure is the pressure a solvent exerts to prevent the inward flow of solvent molecules across a semipermeable membrane due to a difference in solute concentration on either side of the membrane. In essence, the osmotic pressure is the pressure needed to stop the flow of water or osmosis. Osmotic pressure can be thought of as the pressure that. Osmometer Pressure Definition.
From psiberg.com
Osmotic Pressure Determination and Applications Osmometer Pressure Definition Osmotic pressure is the minimum pressure that prevents solvent molecules (water) from flowing through a semipermeable membrane. Osmotic pressure can be thought of as the pressure that would be required to stop water from diffusing through a barrier by. Osmotic pressure is the pressure required to stop the net movement of water across a permeable membrane that divides the solvent. Osmometer Pressure Definition.
From study.com
Osmotic Pressure Definition & Formula Video & Lesson Transcript Osmometer Pressure Definition In other words, it is the pressure of a solvent against a semipermeable membrane that seeks to equalize the concentration of a solution on both sides of the membrane. Osmotic pressure can be thought of as the pressure that would be required to stop water from diffusing through a barrier by. Osmotic pressure is the pressure a solvent exerts to. Osmometer Pressure Definition.
From www.thoughtco.com
Osmotic Pressure and Tonicity Osmometer Pressure Definition In other words, it is the pressure of a solvent against a semipermeable membrane that seeks to equalize the concentration of a solution on both sides of the membrane. In essence, the osmotic pressure is the pressure needed to stop the flow of water or osmosis. Osmotic pressure is the pressure a solvent exerts to prevent the inward flow of. Osmometer Pressure Definition.
From www.slideserve.com
PPT Solutions PowerPoint Presentation, free download ID3588530 Osmometer Pressure Definition In essence, the osmotic pressure is the pressure needed to stop the flow of water or osmosis. Osmotic pressure is the pressure caused by a difference in the amounts of solutes (or molecules) between solutions (or fluids) separated by a semipermeable membrane. Osmotic pressure is the pressure required to stop the net movement of water across a permeable membrane that. Osmometer Pressure Definition.
From ar.inspiredpencil.com
Osmotic Pressure Equation Osmometer Pressure Definition Osmotic pressure is the pressure a solvent exerts to prevent the inward flow of solvent molecules across a semipermeable membrane due to a difference in solute concentration on either side of the membrane. Osmotic pressure is the pressure caused by a difference in the amounts of solutes (or molecules) between solutions (or fluids) separated by a semipermeable membrane. Osmotic pressure. Osmometer Pressure Definition.
From chemistnotes.com
Osmosis/Reverse osmosis/osmotic pressure/Definition/Equation Osmometer Pressure Definition Osmotic pressure is the pressure caused by a difference in the amounts of solutes (or molecules) between solutions (or fluids) separated by a semipermeable membrane. Osmotic pressure can be thought of as the pressure that would be required to stop water from diffusing through a barrier by. Osmotic pressure is the pressure a solvent exerts to prevent the inward flow. Osmometer Pressure Definition.
From thechemistrynotes.com
Osmotic Pressure Definition, Formulas, Laws Osmometer Pressure Definition Osmotic pressure is the minimum pressure that prevents solvent molecules (water) from flowing through a semipermeable membrane. In other words, it is the pressure of a solvent against a semipermeable membrane that seeks to equalize the concentration of a solution on both sides of the membrane. Osmotic pressure can be thought of as the pressure that would be required to. Osmometer Pressure Definition.
From chemistnotes.com
Osmosis/Reverse osmosis/osmotic pressure/Definition/Equation Osmometer Pressure Definition Osmotic pressure can be thought of as the pressure that would be required to stop water from diffusing through a barrier by. Osmotic pressure is the pressure a solvent exerts to prevent the inward flow of solvent molecules across a semipermeable membrane due to a difference in solute concentration on either side of the membrane. In other words, it is. Osmometer Pressure Definition.
From www.sliderbase.com
Colligative Properties of Solutions Presentation Chemistry Osmometer Pressure Definition Osmotic pressure can be thought of as the pressure that would be required to stop water from diffusing through a barrier by. Osmotic pressure is the pressure a solvent exerts to prevent the inward flow of solvent molecules across a semipermeable membrane due to a difference in solute concentration on either side of the membrane. Osmotic pressure is the pressure. Osmometer Pressure Definition.
From www.geeksforgeeks.org
What Is Osmosis? Definition, Types, Osmotic Pressure & Significance Osmometer Pressure Definition In other words, it is the pressure of a solvent against a semipermeable membrane that seeks to equalize the concentration of a solution on both sides of the membrane. Osmotic pressure can be thought of as the pressure that would be required to stop water from diffusing through a barrier by. In essence, the osmotic pressure is the pressure needed. Osmometer Pressure Definition.
From biologynotesonline.com
Osmotic Pressure Definition, Equations, Types, Importance, Examples Osmometer Pressure Definition Osmotic pressure is the pressure caused by a difference in the amounts of solutes (or molecules) between solutions (or fluids) separated by a semipermeable membrane. Osmotic pressure can be thought of as the pressure that would be required to stop water from diffusing through a barrier by. In essence, the osmotic pressure is the pressure needed to stop the flow. Osmometer Pressure Definition.
From biologyideas.com
What is osmosis? Definition, Mechanism & Osmotic Pressure Biology Ideas Osmometer Pressure Definition In essence, the osmotic pressure is the pressure needed to stop the flow of water or osmosis. In other words, it is the pressure of a solvent against a semipermeable membrane that seeks to equalize the concentration of a solution on both sides of the membrane. Osmotic pressure can be thought of as the pressure that would be required to. Osmometer Pressure Definition.
From thechemistrynotes.com
Osmotic Pressure Definition, Formulas, Laws Osmometer Pressure Definition In other words, it is the pressure of a solvent against a semipermeable membrane that seeks to equalize the concentration of a solution on both sides of the membrane. Osmotic pressure is the pressure caused by a difference in the amounts of solutes (or molecules) between solutions (or fluids) separated by a semipermeable membrane. Osmotic pressure is the pressure a. Osmometer Pressure Definition.
From www.vrogue.co
What Is Osmosis Definition Types Osmotic Pressure vrogue.co Osmometer Pressure Definition Osmotic pressure is the pressure caused by a difference in the amounts of solutes (or molecules) between solutions (or fluids) separated by a semipermeable membrane. Osmotic pressure is the pressure required to stop the net movement of water across a permeable membrane that divides the solvent and solution, whereas oncotic pressure is the contribution of colloids to total osmolality. In. Osmometer Pressure Definition.
From surfguppy.com
Osmotic Pressure Surfguppy Chemistry made easy visual learning Osmometer Pressure Definition Osmotic pressure can be thought of as the pressure that would be required to stop water from diffusing through a barrier by. Osmotic pressure is the pressure caused by a difference in the amounts of solutes (or molecules) between solutions (or fluids) separated by a semipermeable membrane. In essence, the osmotic pressure is the pressure needed to stop the flow. Osmometer Pressure Definition.
From sciencenotes.org
Osmotic Pressure Definition, Formula, Examples Osmometer Pressure Definition Osmotic pressure is the pressure required to stop the net movement of water across a permeable membrane that divides the solvent and solution, whereas oncotic pressure is the contribution of colloids to total osmolality. Osmotic pressure can be thought of as the pressure that would be required to stop water from diffusing through a barrier by. Osmotic pressure is the. Osmometer Pressure Definition.
From www.slideserve.com
PPT Chapter 21 PowerPoint Presentation, free download ID1433362 Osmometer Pressure Definition Osmotic pressure is the pressure required to stop the net movement of water across a permeable membrane that divides the solvent and solution, whereas oncotic pressure is the contribution of colloids to total osmolality. In essence, the osmotic pressure is the pressure needed to stop the flow of water or osmosis. In other words, it is the pressure of a. Osmometer Pressure Definition.
From www.vrogue.co
What Is Osmosis Definition Types Osmotic Pressure vrogue.co Osmometer Pressure Definition Osmotic pressure is the minimum pressure that prevents solvent molecules (water) from flowing through a semipermeable membrane. Osmotic pressure is the pressure required to stop the net movement of water across a permeable membrane that divides the solvent and solution, whereas oncotic pressure is the contribution of colloids to total osmolality. In essence, the osmotic pressure is the pressure needed. Osmometer Pressure Definition.
From chem.libretexts.org
13.7 Osmotic Pressure Chemistry LibreTexts Osmometer Pressure Definition Osmotic pressure is the pressure required to stop the net movement of water across a permeable membrane that divides the solvent and solution, whereas oncotic pressure is the contribution of colloids to total osmolality. In other words, it is the pressure of a solvent against a semipermeable membrane that seeks to equalize the concentration of a solution on both sides. Osmometer Pressure Definition.
From www.chemistryland.com
Colligative Properties Osmometer Pressure Definition Osmotic pressure is the pressure caused by a difference in the amounts of solutes (or molecules) between solutions (or fluids) separated by a semipermeable membrane. In other words, it is the pressure of a solvent against a semipermeable membrane that seeks to equalize the concentration of a solution on both sides of the membrane. Osmotic pressure is the pressure a. Osmometer Pressure Definition.
From www.youtube.com
osmosis and osmotic pressure solutions solutions class 12 chemistry Osmometer Pressure Definition In essence, the osmotic pressure is the pressure needed to stop the flow of water or osmosis. Osmotic pressure is the pressure required to stop the net movement of water across a permeable membrane that divides the solvent and solution, whereas oncotic pressure is the contribution of colloids to total osmolality. Osmotic pressure is the minimum pressure that prevents solvent. Osmometer Pressure Definition.