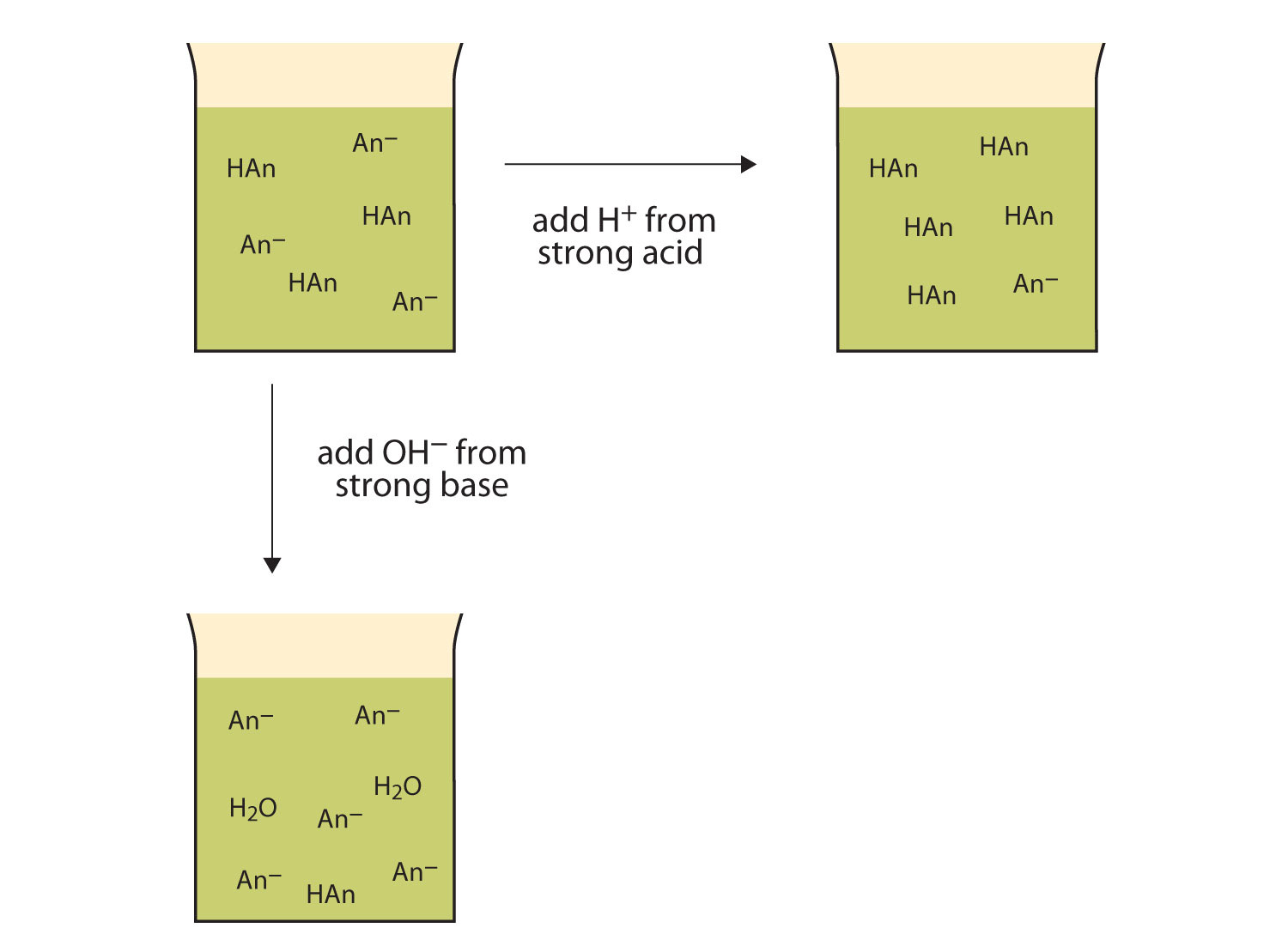
What Happens When You Add A Base To A Buffer . Adding base to the buffer. A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to it. When you add an acid to a buffer, the conjugate base present in the buffer neutralizes it. A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or. When base is added it reacts with the h + ions in the buffer, and this (temporarily) reduces the concentration of the h + ion. It is able to neutralize small amounts of added acid or base, thus. Buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added (figure \ (\pageindex. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. What is a buffer solution? Conversely, when you add a base, the weak acid in the buffer neutralizes the base.
from 2012books.lardbucket.org
What is a buffer solution? It is able to neutralize small amounts of added acid or base, thus. When you add an acid to a buffer, the conjugate base present in the buffer neutralizes it. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Conversely, when you add a base, the weak acid in the buffer neutralizes the base. A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to it. A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or. Buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added (figure \ (\pageindex. Adding base to the buffer. When base is added it reacts with the h + ions in the buffer, and this (temporarily) reduces the concentration of the h + ion.
Buffers
What Happens When You Add A Base To A Buffer A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. When you add an acid to a buffer, the conjugate base present in the buffer neutralizes it. Adding base to the buffer. What is a buffer solution? Buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added (figure \ (\pageindex. It is able to neutralize small amounts of added acid or base, thus. A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to it. A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or. Conversely, when you add a base, the weak acid in the buffer neutralizes the base. When base is added it reacts with the h + ions in the buffer, and this (temporarily) reduces the concentration of the h + ion. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components.
From psiberg.com
Buffer Solutions Principle and Mechanism of their Action PSIBERG What Happens When You Add A Base To A Buffer A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or. Conversely, when you add a base, the weak acid in the buffer neutralizes the base. It is able to neutralize small amounts of added acid or base, thus. When you add an acid. What Happens When You Add A Base To A Buffer.
From www.youtube.com
how adding acid or base changes pH of buffer YouTube What Happens When You Add A Base To A Buffer A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to it. It is able to neutralize small amounts of added acid or base, thus. When you add an acid to a buffer, the conjugate base present in the buffer neutralizes it. Conversely, when you add a base, the. What Happens When You Add A Base To A Buffer.
From www.slideserve.com
PPT Acids Bases Equilibria Part V Buffers PowerPoint Presentation What Happens When You Add A Base To A Buffer What is a buffer solution? A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Conversely, when you add a base, the weak acid in the buffer neutralizes the base. A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added. What Happens When You Add A Base To A Buffer.
From www.slideshare.net
Ch 18 buffers What Happens When You Add A Base To A Buffer What is a buffer solution? A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to it. A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or. Buffer solutions resist a change. What Happens When You Add A Base To A Buffer.
From www.slideserve.com
PPT PART 4 Salt Hydrolysis and Buffer Solutions PowerPoint What Happens When You Add A Base To A Buffer A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or. Buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added (figure \ (\pageindex. Conversely, when you add a base, the weak acid in. What Happens When You Add A Base To A Buffer.
From www.slideserve.com
PPT PART 4 Salt Hydrolysis and Buffer Solutions PowerPoint What Happens When You Add A Base To A Buffer When you add an acid to a buffer, the conjugate base present in the buffer neutralizes it. A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or. A buffer is a solution that can resist ph change upon the addition of an acidic. What Happens When You Add A Base To A Buffer.
From www.osmosis.org
Making buffer solutions Osmosis What Happens When You Add A Base To A Buffer A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to it. Conversely, when you add a base, the weak acid in the buffer neutralizes the base. Buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added (figure \. What Happens When You Add A Base To A Buffer.
From www.slideserve.com
PPT Unit 18 AcidBase Equilibria Buffers & Hydrolysis PowerPoint What Happens When You Add A Base To A Buffer When base is added it reacts with the h + ions in the buffer, and this (temporarily) reduces the concentration of the h + ion. When you add an acid to a buffer, the conjugate base present in the buffer neutralizes it. Buffer solutions resist a change in ph when small amounts of a strong acid or a strong base. What Happens When You Add A Base To A Buffer.
From www.youtube.com
Buffer from weak acid and strong base YouTube What Happens When You Add A Base To A Buffer Buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added (figure \ (\pageindex. Adding base to the buffer. When base is added it reacts with the h + ions in the buffer, and this (temporarily) reduces the concentration of the h + ion. Conversely, when you add a base, the. What Happens When You Add A Base To A Buffer.
From www.chemistrystudent.com
Buffer Solutions (ALevel) ChemistryStudent What Happens When You Add A Base To A Buffer When base is added it reacts with the h + ions in the buffer, and this (temporarily) reduces the concentration of the h + ion. When you add an acid to a buffer, the conjugate base present in the buffer neutralizes it. Buffer solutions resist a change in ph when small amounts of a strong acid or a strong base. What Happens When You Add A Base To A Buffer.
From www.youtube.com
Adding strong base to a buffer solution YouTube What Happens When You Add A Base To A Buffer A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or. When you add an acid to a buffer, the conjugate base present in the buffer neutralizes it. Buffer solutions resist a change in ph when small amounts of a strong acid or a. What Happens When You Add A Base To A Buffer.
From www.slideserve.com
PPT AcidBase Buffers PowerPoint Presentation, free download ID496487 What Happens When You Add A Base To A Buffer A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or. When you add an acid to a buffer, the conjugate base present in the buffer neutralizes it. A buffer is a solution that can resist ph change upon the addition of an acidic. What Happens When You Add A Base To A Buffer.
From www.studocu.com
Practice Question 3 Buffers Solution Buffers what happens when we add What Happens When You Add A Base To A Buffer What is a buffer solution? Buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added (figure \ (\pageindex. A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or. A buffer is a solution. What Happens When You Add A Base To A Buffer.
From classnotes.org.in
Buffer solution and Buffer Action Chemistry, Class 11, Ionic Equilibrium What Happens When You Add A Base To A Buffer It is able to neutralize small amounts of added acid or base, thus. When you add an acid to a buffer, the conjugate base present in the buffer neutralizes it. Conversely, when you add a base, the weak acid in the buffer neutralizes the base. A buffer solution is one which resists changes in ph when small quantities of an. What Happens When You Add A Base To A Buffer.
From 2012books.lardbucket.org
Buffers What Happens When You Add A Base To A Buffer A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Conversely, when you add a base, the weak acid in the buffer neutralizes the base. When you add an acid to a buffer, the conjugate base present in the buffer neutralizes it. Adding base to the buffer. It is able to. What Happens When You Add A Base To A Buffer.
From www.slideserve.com
PPT Chemistry 100 Chapter 17 PowerPoint Presentation, free download What Happens When You Add A Base To A Buffer What is a buffer solution? Conversely, when you add a base, the weak acid in the buffer neutralizes the base. Buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added (figure \ (\pageindex. Adding base to the buffer. When you add an acid to a buffer, the conjugate base present. What Happens When You Add A Base To A Buffer.
From www.slideserve.com
PPT Blood Gases, pH and Buffer system PowerPoint Presentation ID257615 What Happens When You Add A Base To A Buffer What is a buffer solution? Buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added (figure \ (\pageindex. When base is added it reacts with the h + ions in the buffer, and this (temporarily) reduces the concentration of the h + ion. It is able to neutralize small amounts. What Happens When You Add A Base To A Buffer.
From www.slideshare.net
2012 topic 18 2 buffer solutions What Happens When You Add A Base To A Buffer When you add an acid to a buffer, the conjugate base present in the buffer neutralizes it. What is a buffer solution? A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or. A buffer is a solution that can resist ph change upon. What Happens When You Add A Base To A Buffer.
From jackwestin.com
Buffers 2 Acid Base Equilibria MCAT Content What Happens When You Add A Base To A Buffer Buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added (figure \ (\pageindex. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. It is able to neutralize small amounts of added acid or base, thus. What is a buffer solution?. What Happens When You Add A Base To A Buffer.
From www.slideserve.com
PPT Chapter 17 Additional Aspects of Aqueous Equilibria PowerPoint What Happens When You Add A Base To A Buffer What is a buffer solution? When you add an acid to a buffer, the conjugate base present in the buffer neutralizes it. Buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added (figure \ (\pageindex. When base is added it reacts with the h + ions in the buffer, and. What Happens When You Add A Base To A Buffer.
From www.studocu.com
Chap 18 pt2 Adding acid or base to Buffer Chapter 18 AcidBase What Happens When You Add A Base To A Buffer Buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added (figure \ (\pageindex. Conversely, when you add a base, the weak acid in the buffer neutralizes the base. It is able to neutralize small amounts of added acid or base, thus. When base is added it reacts with the h. What Happens When You Add A Base To A Buffer.
From www.slideserve.com
PPT Buffers PowerPoint Presentation, free download ID2372473 What Happens When You Add A Base To A Buffer When you add an acid to a buffer, the conjugate base present in the buffer neutralizes it. A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or. Adding base to the buffer. Conversely, when you add a base, the weak acid in the. What Happens When You Add A Base To A Buffer.
From www.slideserve.com
PPT Buffering Capacity Addition of STRONG Acids or Bases PowerPoint What Happens When You Add A Base To A Buffer What is a buffer solution? Buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added (figure \ (\pageindex. When base is added it reacts with the h + ions in the buffer, and this (temporarily) reduces the concentration of the h + ion. When you add an acid to a. What Happens When You Add A Base To A Buffer.
From slideplayer.com
Chapter 16 Acids and Bases ppt download What Happens When You Add A Base To A Buffer Adding base to the buffer. It is able to neutralize small amounts of added acid or base, thus. When you add an acid to a buffer, the conjugate base present in the buffer neutralizes it. A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to it. Conversely, when. What Happens When You Add A Base To A Buffer.
From labpedia.net
Acidbase Balance Part 3 Respiratory Acidosis and Respiratory What Happens When You Add A Base To A Buffer When you add an acid to a buffer, the conjugate base present in the buffer neutralizes it. What is a buffer solution? Conversely, when you add a base, the weak acid in the buffer neutralizes the base. When base is added it reacts with the h + ions in the buffer, and this (temporarily) reduces the concentration of the h. What Happens When You Add A Base To A Buffer.
From www.youtube.com
Acids, Bases, pH, Buffers YouTube What Happens When You Add A Base To A Buffer A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. What is a buffer solution? When base is added it reacts with the h + ions in the buffer, and this (temporarily) reduces the concentration of the h + ion. Buffer solutions resist a change in ph when small amounts of. What Happens When You Add A Base To A Buffer.
From www.slideserve.com
PPT Buffer Solutions PowerPoint Presentation, free download ID5799007 What Happens When You Add A Base To A Buffer A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. When you add an acid to a buffer, the conjugate base present in the buffer neutralizes it. It is able to neutralize small amounts of added acid or base, thus. Adding base to the buffer. What is a buffer solution? Conversely,. What Happens When You Add A Base To A Buffer.
From www.slideserve.com
PPT Buffer solutions PowerPoint Presentation, free download ID2813415 What Happens When You Add A Base To A Buffer What is a buffer solution? A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to it. When base is added it reacts with the h + ions in the buffer, and this (temporarily) reduces the concentration of the h + ion. Adding base to the buffer. A mixture. What Happens When You Add A Base To A Buffer.
From www.slideserve.com
PPT Chemistry 100 Chapter 17 PowerPoint Presentation, free download What Happens When You Add A Base To A Buffer What is a buffer solution? A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added (figure \ (\pageindex. Conversely, when you add a base, the weak acid in the buffer neutralizes. What Happens When You Add A Base To A Buffer.
From www.hopaxfc.com
How can Biological Buffer maintain the pH value? Blog Hopax Fine What Happens When You Add A Base To A Buffer Buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added (figure \ (\pageindex. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. When base is added it reacts with the h + ions in the buffer, and this (temporarily) reduces. What Happens When You Add A Base To A Buffer.
From chem.libretexts.org
14.6 Buffers Chemistry LibreTexts What Happens When You Add A Base To A Buffer A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Adding base to the buffer. It is able to neutralize small amounts of added acid or base, thus. What is a buffer solution? When base is added it reacts with the h + ions in the buffer, and this (temporarily) reduces. What Happens When You Add A Base To A Buffer.
From mavink.com
Acids Bases And Buffers What Happens When You Add A Base To A Buffer Adding base to the buffer. A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or. What is a buffer solution? When you add an acid to a buffer, the conjugate base present in the buffer neutralizes it. It is able to neutralize small. What Happens When You Add A Base To A Buffer.
From www.ck12.org
Buffer Solutions Overview ( Video ) Chemistry CK12 Foundation What Happens When You Add A Base To A Buffer Adding base to the buffer. When you add an acid to a buffer, the conjugate base present in the buffer neutralizes it. When base is added it reacts with the h + ions in the buffer, and this (temporarily) reduces the concentration of the h + ion. Buffer solutions resist a change in ph when small amounts of a strong. What Happens When You Add A Base To A Buffer.
From www.slideserve.com
PPT AcidBase Buffers PowerPoint Presentation ID496487 What Happens When You Add A Base To A Buffer When you add an acid to a buffer, the conjugate base present in the buffer neutralizes it. A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to it. What is a buffer solution? Buffer solutions resist a change in ph when small amounts of a strong acid or. What Happens When You Add A Base To A Buffer.
From www.slideserve.com
PPT PART 4 Salt Hydrolysis and Buffer Solutions PowerPoint What Happens When You Add A Base To A Buffer When base is added it reacts with the h + ions in the buffer, and this (temporarily) reduces the concentration of the h + ion. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Buffer solutions resist a change in ph when small amounts of a strong acid or a. What Happens When You Add A Base To A Buffer.