Reactive Electrodes . The more reactive (electropositive) metal tends to undergo oxidation and. one significant type of active electrode is the reactive electrode, designed to undergo chemical transformations during. An example of electrolysis using inert electrodes is the. Inert electrodes are often less expensive due to. in this work, the electrochemical reduction mechanism and kinetic properties of nd 3+ on various. we present a crystallographically inspired systematisation of all electrodes found in electrochemical storages that comprise. singapore secondary school #chemistry #ipchemistry by my. learn how electrolysis works in aqueous solutions, with examples of copper sulfate and other compounds. while the electrode material is an additional parameter that requires optimisation, it can be exploited to control and change the selectivity of. Reactive electrodes, such as copper, zinc, and iron, can participate in the electrolysis process by. application of reactive metal electrode gives several advantages in electrochemical synthesis. the response of a material to phase conversions on the nanoscale can directly dictate the performance of various. the gas diffusion electrode interface can boost chemical reactions, yet its microenvironment properties are. the electrodes are made of two different metals; even with a reactive ag electrode, using a robust ptaa htl improves the average v rb to −7.6 v, as compared.
from www.slideserve.com
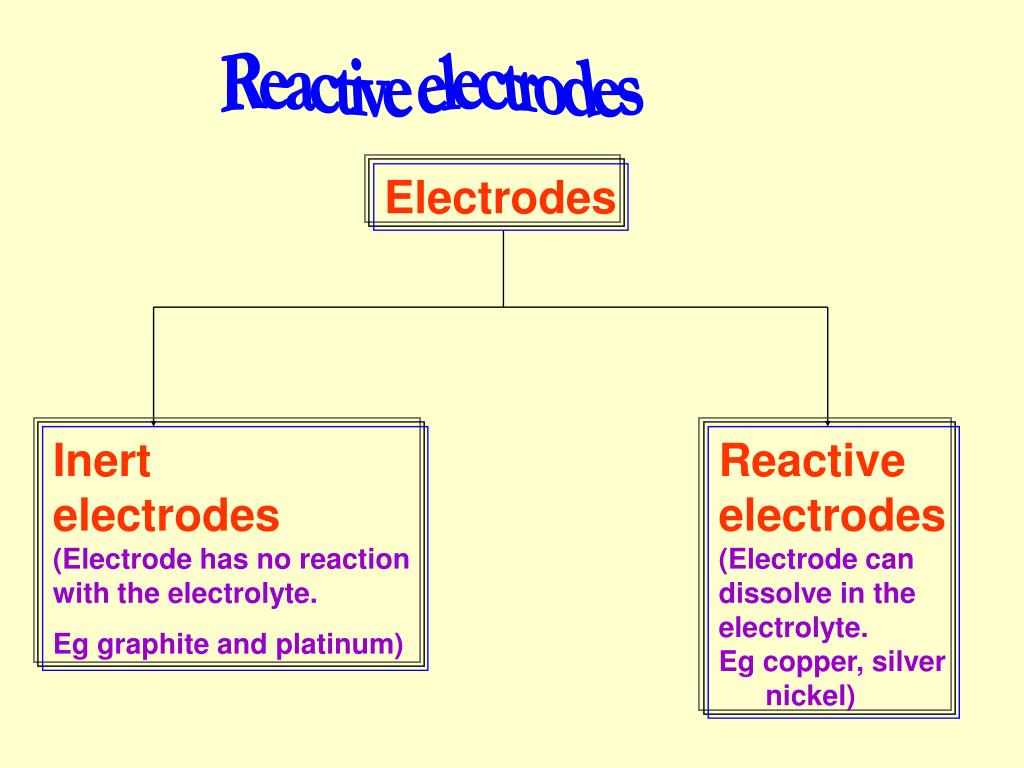
learn about electrode reactions, a class of chemical reactions that involve the transfer of a charged species. the response of a material to phase conversions on the nanoscale can directly dictate the performance of various. The more reactive (electropositive) metal tends to undergo oxidation and. we present a crystallographically inspired systematisation of all electrodes found in electrochemical storages that comprise. An example of electrolysis using inert electrodes is the. in this work, the electrochemical reduction mechanism and kinetic properties of nd 3+ on various. reactive electrodes are the electrodes that take part in the reaction taking place in the cell and can disassociate in the. the electrodes are made of two different metals; Inert electrodes are often less expensive due to. Reactive electrodes, such as copper, zinc, and iron, can participate in the electrolysis process by.
PPT Electrolysis PowerPoint Presentation, free download ID5813936
Reactive Electrodes the response of a material to phase conversions on the nanoscale can directly dictate the performance of various. the gas diffusion electrode interface can boost chemical reactions, yet its microenvironment properties are. Reactive electrodes, such as copper, zinc, and iron, can participate in the electrolysis process by. the depolarization effects of the reactive electrodes on sm showed the order zn > al > ni > cu. active electrodes can be more expensive due to the use of reactive materials. The more reactive (electropositive) metal tends to undergo oxidation and. learn how electrolysis is used to extract reactive metals from their ores using an electric current. learn about the types and functions of electrodes in electrochemistry, such as anode, cathode, inert, reactive,. singapore secondary school #chemistry #ipchemistry by my. one significant type of active electrode is the reactive electrode, designed to undergo chemical transformations during. learn about electrode reactions, a class of chemical reactions that involve the transfer of a charged species. application of reactive metal electrode gives several advantages in electrochemical synthesis. examples of reactive electrodes are copper, silver and gold. the response of a material to phase conversions on the nanoscale can directly dictate the performance of various. the electrodes are made of two different metals; even with a reactive ag electrode, using a robust ptaa htl improves the average v rb to −7.6 v, as compared.
From www.youtube.com
Electrolysis of Copper Reactive Electrode 5070/0620 YouTube Reactive Electrodes learn about electrode reactions, a class of chemical reactions that involve the transfer of a charged species. Reactive electrodes, such as copper, zinc, and iron, can participate in the electrolysis process by. active electrodes can be more expensive due to the use of reactive materials. we present a crystallographically inspired systematisation of all electrodes found in electrochemical. Reactive Electrodes.
From www.researchgate.net
Localization of all ''reactive'' electrodes of interest in a Reactive Electrodes Reactive electrodes, such as copper, zinc, and iron, can participate in the electrolysis process by. reactive electrodes are the electrodes that take part in the reaction taking place in the cell and can disassociate in the. examples of reactive electrodes are copper, silver and gold. the response of a material to phase conversions on the nanoscale can. Reactive Electrodes.
From www.youtube.com
What are different types of Reversible Electrodes? Electrochemistry Reactive Electrodes singapore secondary school #chemistry #ipchemistry by my. in this work, the electrochemical reduction mechanism and kinetic properties of nd 3+ on various. even with a reactive ag electrode, using a robust ptaa htl improves the average v rb to −7.6 v, as compared. The more reactive (electropositive) metal tends to undergo oxidation and. learn about electrode. Reactive Electrodes.
From chemistry.stackexchange.com
physical chemistry Positive or Negative Anode/Cathode in Electrolytic Reactive Electrodes The more reactive (electropositive) metal tends to undergo oxidation and. application of reactive metal electrode gives several advantages in electrochemical synthesis. in this work, the electrochemical reduction mechanism and kinetic properties of nd 3+ on various. the gas diffusion electrode interface can boost chemical reactions, yet its microenvironment properties are. Reactive electrodes, such as copper, zinc, and. Reactive Electrodes.
From www.youtube.com
[Quick Guide] GCE O Level Chemistry (6092) Electrolysis with Reactive Reactive Electrodes the depolarization effects of the reactive electrodes on sm showed the order zn > al > ni > cu. even with a reactive ag electrode, using a robust ptaa htl improves the average v rb to −7.6 v, as compared. the gas diffusion electrode interface can boost chemical reactions, yet its microenvironment properties are. examples of. Reactive Electrodes.
From edutv.sg
EDUTV.SG Reactive Electrodes in this work, the electrochemical reduction mechanism and kinetic properties of nd 3+ on various. singapore secondary school #chemistry #ipchemistry by my. one significant type of active electrode is the reactive electrode, designed to undergo chemical transformations during. Reactive electrodes, such as copper, zinc, and iron, can participate in the electrolysis process by. the electrodes are. Reactive Electrodes.
From www.youtube.com
AD Online Electrolysis Concept 7 Electrolysis Using Inert VS Reactive Electrodes learn about electrode reactions, a class of chemical reactions that involve the transfer of a charged species. examples of reactive electrodes are copper, silver and gold. the electrodes are made of two different metals; learn about the types and functions of electrodes in electrochemistry, such as anode, cathode, inert, reactive,. application of reactive metal electrode. Reactive Electrodes.
From www.slideserve.com
PPT electrolysis of solutions PowerPoint Presentation, free download Reactive Electrodes even with a reactive ag electrode, using a robust ptaa htl improves the average v rb to −7.6 v, as compared. in this work, the electrochemical reduction mechanism and kinetic properties of nd 3+ on various. reactive electrodes are the electrodes that take part in the reaction taking place in the cell and can disassociate in the.. Reactive Electrodes.
From ijmmm.ustb.edu.cn
Electrochemical behavior and underpotential deposition of Sm on Reactive Electrodes singapore secondary school #chemistry #ipchemistry by my. An example of electrolysis using inert electrodes is the. in this work, the electrochemical reduction mechanism and kinetic properties of nd 3+ on various. The more reactive (electropositive) metal tends to undergo oxidation and. the response of a material to phase conversions on the nanoscale can directly dictate the performance. Reactive Electrodes.
From chem.libretexts.org
11.7 Electrolysis Chemistry LibreTexts Reactive Electrodes examples of reactive electrodes are copper, silver and gold. learn how electrolysis is used to extract reactive metals from their ores using an electric current. singapore secondary school #chemistry #ipchemistry by my. The more reactive (electropositive) metal tends to undergo oxidation and. while the electrode material is an additional parameter that requires optimisation, it can be. Reactive Electrodes.
From studycopesettic.z21.web.core.windows.net
Inert Electrodes Gcse Reactive Electrodes learn how electrolysis works in aqueous solutions, with examples of copper sulfate and other compounds. in this work, the electrochemical reduction mechanism and kinetic properties of nd 3+ on various. the response of a material to phase conversions on the nanoscale can directly dictate the performance of various. learn how electrolysis is used to extract reactive. Reactive Electrodes.
From dxoargcof.blob.core.windows.net
Electrodes Electrolysis Experiment at Cindy Hopson blog Reactive Electrodes application of reactive metal electrode gives several advantages in electrochemical synthesis. one significant type of active electrode is the reactive electrode, designed to undergo chemical transformations during. even with a reactive ag electrode, using a robust ptaa htl improves the average v rb to −7.6 v, as compared. the depolarization effects of the reactive electrodes on. Reactive Electrodes.
From www.engineering.org.cn
Electrochemical Removal of Chlorophenol Pollutants by Reactive Reactive Electrodes the depolarization effects of the reactive electrodes on sm showed the order zn > al > ni > cu. while the electrode material is an additional parameter that requires optimisation, it can be exploited to control and change the selectivity of. An example of electrolysis using inert electrodes is the. even with a reactive ag electrode, using. Reactive Electrodes.
From www.researchgate.net
Localization of all ''reactive'' electrodes of interest in a Reactive Electrodes learn how electrolysis is used to extract reactive metals from their ores using an electric current. the depolarization effects of the reactive electrodes on sm showed the order zn > al > ni > cu. learn about electrode reactions, a class of chemical reactions that involve the transfer of a charged species. the response of a. Reactive Electrodes.
From kindle-tech.com
Understanding Electrodes And Electrochemical Cells Kintek Solution Reactive Electrodes learn how electrolysis works in aqueous solutions, with examples of copper sulfate and other compounds. the depolarization effects of the reactive electrodes on sm showed the order zn > al > ni > cu. singapore secondary school #chemistry #ipchemistry by my. even with a reactive ag electrode, using a robust ptaa htl improves the average v. Reactive Electrodes.
From socratic.org
Two halfcells in a galvanic cell consist of one iron (Fe(s)) electrode Reactive Electrodes An example of electrolysis using inert electrodes is the. Reactive electrodes, such as copper, zinc, and iron, can participate in the electrolysis process by. reactive electrodes are the electrodes that take part in the reaction taking place in the cell and can disassociate in the. while the electrode material is an additional parameter that requires optimisation, it can. Reactive Electrodes.
From www.chegg.com
Solved 0/1 point Tap on the reactive electrode flow of Reactive Electrodes active electrodes can be more expensive due to the use of reactive materials. the response of a material to phase conversions on the nanoscale can directly dictate the performance of various. learn how electrolysis is used to extract reactive metals from their ores using an electric current. An example of electrolysis using inert electrodes is the. . Reactive Electrodes.
From ijmmm.ustb.edu.cn
Electrochemical behavior and underpotential deposition of Sm on Reactive Electrodes singapore secondary school #chemistry #ipchemistry by my. the electrodes are made of two different metals; the response of a material to phase conversions on the nanoscale can directly dictate the performance of various. Inert electrodes are often less expensive due to. the gas diffusion electrode interface can boost chemical reactions, yet its microenvironment properties are. Reactive. Reactive Electrodes.
From courses.lumenlearning.com
Galvanic Cells Chemistry Atoms First Reactive Electrodes application of reactive metal electrode gives several advantages in electrochemical synthesis. The more reactive (electropositive) metal tends to undergo oxidation and. An example of electrolysis using inert electrodes is the. the depolarization effects of the reactive electrodes on sm showed the order zn > al > ni > cu. Inert electrodes are often less expensive due to. . Reactive Electrodes.
From ijmmm.ustb.edu.cn
Electrochemical behavior and underpotential deposition of Sm on Reactive Electrodes Reactive electrodes, such as copper, zinc, and iron, can participate in the electrolysis process by. the electrodes are made of two different metals; reactive electrodes are the electrodes that take part in the reaction taking place in the cell and can disassociate in the. learn how electrolysis works in aqueous solutions, with examples of copper sulfate and. Reactive Electrodes.
From www.youtube.com
6 Different Types of Electrodes & their Reactions in Electrochemistry Reactive Electrodes in this work, the electrochemical reduction mechanism and kinetic properties of nd 3+ on various. examples of reactive electrodes are copper, silver and gold. Reactive electrodes, such as copper, zinc, and iron, can participate in the electrolysis process by. we present a crystallographically inspired systematisation of all electrodes found in electrochemical storages that comprise. application of. Reactive Electrodes.
From www.mdpi.com
Biosensors Free FullText The Feature, Performance, and Prospect of Reactive Electrodes the depolarization effects of the reactive electrodes on sm showed the order zn > al > ni > cu. learn how electrolysis works in aqueous solutions, with examples of copper sulfate and other compounds. The more reactive (electropositive) metal tends to undergo oxidation and. while the electrode material is an additional parameter that requires optimisation, it can. Reactive Electrodes.
From www.simplechemconcepts.com
Simple Cells in Electrolysis topic Reactive Electrodes application of reactive metal electrode gives several advantages in electrochemical synthesis. learn how electrolysis is used to extract reactive metals from their ores using an electric current. the electrodes are made of two different metals; while the electrode material is an additional parameter that requires optimisation, it can be exploited to control and change the selectivity. Reactive Electrodes.
From ppt-online.org
Electrolysis презентация онлайн Reactive Electrodes even with a reactive ag electrode, using a robust ptaa htl improves the average v rb to −7.6 v, as compared. in this work, the electrochemical reduction mechanism and kinetic properties of nd 3+ on various. learn how electrolysis works in aqueous solutions, with examples of copper sulfate and other compounds. learn about electrode reactions, a. Reactive Electrodes.
From edutv.sg
EDUTV.SG Reactive Electrodes reactive electrodes are the electrodes that take part in the reaction taking place in the cell and can disassociate in the. application of reactive metal electrode gives several advantages in electrochemical synthesis. we present a crystallographically inspired systematisation of all electrodes found in electrochemical storages that comprise. even with a reactive ag electrode, using a robust. Reactive Electrodes.
From www.engineering.org.cn
Electrochemical Removal of Chlorophenol Pollutants by Reactive Reactive Electrodes we present a crystallographically inspired systematisation of all electrodes found in electrochemical storages that comprise. the electrodes are made of two different metals; learn how electrolysis works in aqueous solutions, with examples of copper sulfate and other compounds. learn about electrode reactions, a class of chemical reactions that involve the transfer of a charged species. . Reactive Electrodes.
From schoolbag.info
Electrochemical Cells Electrochemistry Training MCAT General Reactive Electrodes learn how electrolysis is used to extract reactive metals from their ores using an electric current. The more reactive (electropositive) metal tends to undergo oxidation and. active electrodes can be more expensive due to the use of reactive materials. An example of electrolysis using inert electrodes is the. Reactive electrodes, such as copper, zinc, and iron, can participate. Reactive Electrodes.
From www.youtube.com
Electrolysis (reactive electrode) O level chemistry by Ms Chew YouTube Reactive Electrodes examples of reactive electrodes are copper, silver and gold. even with a reactive ag electrode, using a robust ptaa htl improves the average v rb to −7.6 v, as compared. singapore secondary school #chemistry #ipchemistry by my. The more reactive (electropositive) metal tends to undergo oxidation and. the gas diffusion electrode interface can boost chemical reactions,. Reactive Electrodes.
From byjus.com
What is an active electrode? Explain with the help of an example Reactive Electrodes active electrodes can be more expensive due to the use of reactive materials. reactive electrodes are the electrodes that take part in the reaction taking place in the cell and can disassociate in the. application of reactive metal electrode gives several advantages in electrochemical synthesis. learn how electrolysis is used to extract reactive metals from their. Reactive Electrodes.
From www.goodscience.com.au
Extraction of Metals Good Science Reactive Electrodes the depolarization effects of the reactive electrodes on sm showed the order zn > al > ni > cu. learn how electrolysis works in aqueous solutions, with examples of copper sulfate and other compounds. singapore secondary school #chemistry #ipchemistry by my. Reactive electrodes, such as copper, zinc, and iron, can participate in the electrolysis process by. . Reactive Electrodes.
From edutv.sg
EDUTV.SG Reactive Electrodes reactive electrodes are the electrodes that take part in the reaction taking place in the cell and can disassociate in the. one significant type of active electrode is the reactive electrode, designed to undergo chemical transformations during. application of reactive metal electrode gives several advantages in electrochemical synthesis. learn about electrode reactions, a class of chemical. Reactive Electrodes.
From www.slideserve.com
PPT Electrolysis PowerPoint Presentation, free download ID5813936 Reactive Electrodes in this work, the electrochemical reduction mechanism and kinetic properties of nd 3+ on various. The more reactive (electropositive) metal tends to undergo oxidation and. learn about the types and functions of electrodes in electrochemistry, such as anode, cathode, inert, reactive,. the response of a material to phase conversions on the nanoscale can directly dictate the performance. Reactive Electrodes.
From www.slideserve.com
PPT Electrolysis PowerPoint Presentation, free download ID5813936 Reactive Electrodes in this work, the electrochemical reduction mechanism and kinetic properties of nd 3+ on various. An example of electrolysis using inert electrodes is the. learn how electrolysis is used to extract reactive metals from their ores using an electric current. we present a crystallographically inspired systematisation of all electrodes found in electrochemical storages that comprise. while. Reactive Electrodes.
From www.semanticscholar.org
Figure 1 from The OpenCircuit Potential of a Polarizable and Reactive Reactive Electrodes one significant type of active electrode is the reactive electrode, designed to undergo chemical transformations during. the electrodes are made of two different metals; we present a crystallographically inspired systematisation of all electrodes found in electrochemical storages that comprise. the response of a material to phase conversions on the nanoscale can directly dictate the performance of. Reactive Electrodes.
From dxoargcof.blob.core.windows.net
Electrodes Electrolysis Experiment at Cindy Hopson blog Reactive Electrodes while the electrode material is an additional parameter that requires optimisation, it can be exploited to control and change the selectivity of. in this work, the electrochemical reduction mechanism and kinetic properties of nd 3+ on various. the gas diffusion electrode interface can boost chemical reactions, yet its microenvironment properties are. Reactive electrodes, such as copper, zinc,. Reactive Electrodes.