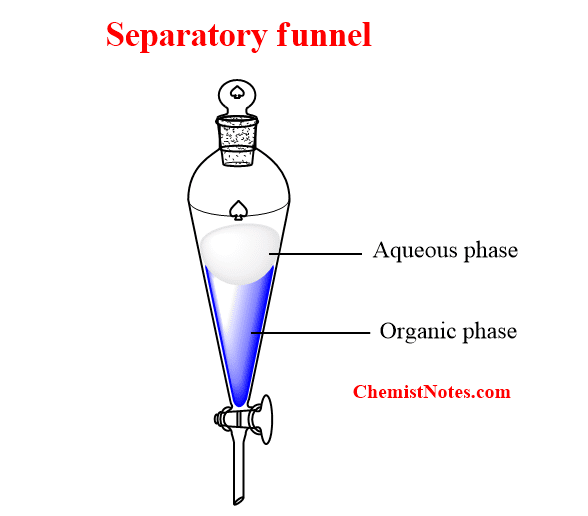
Separatory Funnel Water Extraction . learn how to use water and an organic solvent to separate polar and nonpolar compounds by solvent partitioning. learn the basics of using a separatory funnel for extraction and washing reactions in synthetic chemistry. Find out how to check the. separating funnels are commonly used in a variety of industrial applications where it is necessary to separate immiscible liquids. Find out which layer contains the compound of interest,. learn how to use a separating funnel for solvent extractions in organic chemistry with this slideshow. proper use of a separatory funnel requires timing and dexterity. learn how to use a separatory funnel to extract and wash organic products from aqueous solutions. use of a separatory funnel (quick link to video : when working up this reaction, the resulting mixture is often poured into a separatory funnel along with some water. Extraction) the separatory funnel is a tapered vessel with a stopcock at the. learn how to use partition or distribution coefficient (k) to calculate the amount of solute extracted from a solution using a. learn how to use a separatory funnel (sep funnel) to separate immiscible liquids, such as iodine in water and dichloromethane. First, you should recognize that the purpose is. learn how to identify the aqueous and organic layers in a separatory funnel based on density, solvent properties, and water test.
from chemistnotes.com
thus, the solution containing the solute must be collected and returned to the separatory funnel (or remain in the funnel if this. learn how to use a separating funnel for solvent extractions in organic chemistry with this slideshow. Extraction) the separatory funnel is a tapered vessel with a stopcock at the. learn how to identify the aqueous and organic layers in a separatory funnel based on density, solvent properties, and water test. use of a separatory funnel (quick link to video : Find out how to check the. when working up this reaction, the resulting mixture is often poured into a separatory funnel along with some water. learn how to perform an extraction using a separatory funnel in. proper use of a separatory funnel requires timing and dexterity. learn how to use a separatory funnel to extract and wash organic products from aqueous solutions.
Solvent extraction Principle, easy process, application Chemistry Notes
Separatory Funnel Water Extraction learn how to use water and an organic solvent to separate polar and nonpolar compounds by solvent partitioning. learn how to use separatory funnels to perform single and multiple extractions of organic compounds from aqueous. First, you should recognize that the purpose is. learn how to perform an extraction using a separatory funnel in. learn how to identify the aqueous and organic layers in a separatory funnel based on density, solvent properties, and water test. learn how to use partition or distribution coefficient (k) to calculate the amount of solute extracted from a solution using a. Find out how to check the. separating funnels are commonly used in a variety of industrial applications where it is necessary to separate immiscible liquids. thus, the solution containing the solute must be collected and returned to the separatory funnel (or remain in the funnel if this. learn the basics of using a separatory funnel for extraction and washing reactions in synthetic chemistry. when working up this reaction, the resulting mixture is often poured into a separatory funnel along with some water. learn the techniques and proper use of a separatory funnel, which is a piece of lab glassware used to separate 2 immiscible. learn how to use water and an organic solvent to separate polar and nonpolar compounds by solvent partitioning. learn how to use a separating funnel for solvent extractions in organic chemistry with this slideshow. use of a separatory funnel (quick link to video : learn how to use a separatory funnel (sep funnel) to separate immiscible liquids, such as iodine in water and dichloromethane.
From www.homesciencetools.com
500 ml Separatory Funnel squibb style Separatory Funnel Water Extraction learn how to use a separatory funnel to extract and wash organic products from aqueous solutions. learn how to use separatory funnels to perform single and multiple extractions of organic compounds from aqueous. Find out how to check the. proper use of a separatory funnel requires timing and dexterity. learn how to use a separatory funnel. Separatory Funnel Water Extraction.
From www.thesciencehive.co.uk
Organic Synthesis* — the science sauce Separatory Funnel Water Extraction learn the techniques and proper use of a separatory funnel, which is a piece of lab glassware used to separate 2 immiscible. First, you should recognize that the purpose is. learn how to use a separating funnel for solvent extractions in organic chemistry with this slideshow. when working up this reaction, the resulting mixture is often poured. Separatory Funnel Water Extraction.
From www.youtube.com
ALevel PreLab Video for Using a Separating Funnel YouTube Separatory Funnel Water Extraction when working up this reaction, the resulting mixture is often poured into a separatory funnel along with some water. learn how to use partition or distribution coefficient (k) to calculate the amount of solute extracted from a solution using a. Extraction) the separatory funnel is a tapered vessel with a stopcock at the. learn the techniques and. Separatory Funnel Water Extraction.
From dxokzamrg.blob.core.windows.net
Separatory Funnel Examples at Paula Malloy blog Separatory Funnel Water Extraction thus, the solution containing the solute must be collected and returned to the separatory funnel (or remain in the funnel if this. Find out how to check the. Extraction) the separatory funnel is a tapered vessel with a stopcock at the. learn how to use partition or distribution coefficient (k) to calculate the amount of solute extracted from. Separatory Funnel Water Extraction.
From www.researchgate.net
Photographs of separatory funnel containing chloroform and hydrolysed Separatory Funnel Water Extraction learn how to use a separating funnel for solvent extractions in organic chemistry with this slideshow. learn how to use water and an organic solvent to separate polar and nonpolar compounds by solvent partitioning. learn the techniques and proper use of a separatory funnel, which is a piece of lab glassware used to separate 2 immiscible. . Separatory Funnel Water Extraction.
From scientificglassdesign.com
Separatory Funnel « Scientific Glass Design Separatory Funnel Water Extraction thus, the solution containing the solute must be collected and returned to the separatory funnel (or remain in the funnel if this. Find out which layer contains the compound of interest,. learn how to use separatory funnels to perform single and multiple extractions of organic compounds from aqueous. proper use of a separatory funnel requires timing and. Separatory Funnel Water Extraction.
From hydrobuilder.com
Across International 5 Liter Glass Separatory Funnel Kit with All PTFE Separatory Funnel Water Extraction learn how to use a separatory funnel to extract and wash organic products from aqueous solutions. learn how to use a separating funnel for solvent extractions in organic chemistry with this slideshow. First, you should recognize that the purpose is. Extraction) the separatory funnel is a tapered vessel with a stopcock at the. learn how to identify. Separatory Funnel Water Extraction.
From www.youtube.com
Separating Funnel How to Use Separating Funnel In LiquidLiquid Separatory Funnel Water Extraction learn the basics of using a separatory funnel for extraction and washing reactions in synthetic chemistry. learn how to use partition or distribution coefficient (k) to calculate the amount of solute extracted from a solution using a. use of a separatory funnel (quick link to video : learn the techniques and proper use of a separatory. Separatory Funnel Water Extraction.
From eduinput.com
Separatory FunnelPrinciple, Parts, Types, Sizes, and Applications Separatory Funnel Water Extraction proper use of a separatory funnel requires timing and dexterity. learn how to use a separatory funnel to extract and wash organic products from aqueous solutions. use of a separatory funnel (quick link to video : Find out which layer contains the compound of interest,. learn how to use separatory funnels to perform single and multiple. Separatory Funnel Water Extraction.
From www.youtube.com
Workup using separatory funnel (LiquidLiquid extraction) YouTube Separatory Funnel Water Extraction learn the techniques and proper use of a separatory funnel, which is a piece of lab glassware used to separate 2 immiscible. Find out which layer contains the compound of interest,. learn how to perform an extraction using a separatory funnel in. learn how to identify the aqueous and organic layers in a separatory funnel based on. Separatory Funnel Water Extraction.
From dxokzamrg.blob.core.windows.net
Separatory Funnel Examples at Paula Malloy blog Separatory Funnel Water Extraction learn how to use partition or distribution coefficient (k) to calculate the amount of solute extracted from a solution using a. First, you should recognize that the purpose is. separating funnels are commonly used in a variety of industrial applications where it is necessary to separate immiscible liquids. learn the techniques and proper use of a separatory. Separatory Funnel Water Extraction.
From www.slideshare.net
Extraction Separatory Funnel Water Extraction learn how to use a separatory funnel to extract and wash organic products from aqueous solutions. Find out how to check the. learn how to use partition or distribution coefficient (k) to calculate the amount of solute extracted from a solution using a. when working up this reaction, the resulting mixture is often poured into a separatory. Separatory Funnel Water Extraction.
From www.researchgate.net
Diagrammatic illustration of liquidliquid extraction (adapted from Separatory Funnel Water Extraction Find out which layer contains the compound of interest,. First, you should recognize that the purpose is. learn how to use a separating funnel for solvent extractions in organic chemistry with this slideshow. when working up this reaction, the resulting mixture is often poured into a separatory funnel along with some water. learn the techniques and proper. Separatory Funnel Water Extraction.
From www.researchgate.net
Extraction of the product from water (A and B) (A) water and organic Separatory Funnel Water Extraction when working up this reaction, the resulting mixture is often poured into a separatory funnel along with some water. learn the techniques and proper use of a separatory funnel, which is a piece of lab glassware used to separate 2 immiscible. Find out how to check the. learn how to use water and an organic solvent to. Separatory Funnel Water Extraction.
From www.chem.ucla.edu
How to use the separatory funnel Separatory Funnel Water Extraction First, you should recognize that the purpose is. learn how to use a separating funnel for solvent extractions in organic chemistry with this slideshow. use of a separatory funnel (quick link to video : Extraction) the separatory funnel is a tapered vessel with a stopcock at the. learn how to perform an extraction using a separatory funnel. Separatory Funnel Water Extraction.
From eduinput.com
Separatory FunnelPrinciple, Parts, Types, Sizes, and Applications Separatory Funnel Water Extraction learn how to use a separatory funnel (sep funnel) to separate immiscible liquids, such as iodine in water and dichloromethane. learn how to use water and an organic solvent to separate polar and nonpolar compounds by solvent partitioning. Find out which layer contains the compound of interest,. learn how to use partition or distribution coefficient (k) to. Separatory Funnel Water Extraction.
From www.pngwing.com
Chemistry Separatory funnel Separation process Extraction Decantation Separatory Funnel Water Extraction thus, the solution containing the solute must be collected and returned to the separatory funnel (or remain in the funnel if this. Extraction) the separatory funnel is a tapered vessel with a stopcock at the. learn how to identify the aqueous and organic layers in a separatory funnel based on density, solvent properties, and water test. learn. Separatory Funnel Water Extraction.
From themasterchemistry.com
Separating FunnelWorking Principle And Uses Separatory Funnel Water Extraction learn how to use a separatory funnel (sep funnel) to separate immiscible liquids, such as iodine in water and dichloromethane. Find out how to check the. thus, the solution containing the solute must be collected and returned to the separatory funnel (or remain in the funnel if this. learn how to use water and an organic solvent. Separatory Funnel Water Extraction.
From www.slideserve.com
PPT Separation PowerPoint Presentation ID2857210 Separatory Funnel Water Extraction learn how to use a separatory funnel (sep funnel) to separate immiscible liquids, such as iodine in water and dichloromethane. thus, the solution containing the solute must be collected and returned to the separatory funnel (or remain in the funnel if this. learn how to use separatory funnels to perform single and multiple extractions of organic compounds. Separatory Funnel Water Extraction.
From www.animalia-life.club
Separating Funnel Labelled Diagram Separatory Funnel Water Extraction learn how to use water and an organic solvent to separate polar and nonpolar compounds by solvent partitioning. proper use of a separatory funnel requires timing and dexterity. learn how to use a separatory funnel to extract and wash organic products from aqueous solutions. First, you should recognize that the purpose is. learn how to use. Separatory Funnel Water Extraction.
From www.youtube.com
Liquid liquid extraction LLE with a separatory funnel YouTube Separatory Funnel Water Extraction learn the basics of using a separatory funnel for extraction and washing reactions in synthetic chemistry. Extraction) the separatory funnel is a tapered vessel with a stopcock at the. learn how to use water and an organic solvent to separate polar and nonpolar compounds by solvent partitioning. proper use of a separatory funnel requires timing and dexterity.. Separatory Funnel Water Extraction.
From www.aurorabiomed.com
LiquidLiquid Extraction vs. SolidPhase Extraction Separatory Funnel Water Extraction separating funnels are commonly used in a variety of industrial applications where it is necessary to separate immiscible liquids. learn how to use a separating funnel for solvent extractions in organic chemistry with this slideshow. proper use of a separatory funnel requires timing and dexterity. thus, the solution containing the solute must be collected and returned. Separatory Funnel Water Extraction.
From chem.libretexts.org
4.6 StepbyStep Procedures For Extractions Chemistry LibreTexts Separatory Funnel Water Extraction learn how to use separatory funnels to perform single and multiple extractions of organic compounds from aqueous. proper use of a separatory funnel requires timing and dexterity. learn how to identify the aqueous and organic layers in a separatory funnel based on density, solvent properties, and water test. learn the basics of using a separatory funnel. Separatory Funnel Water Extraction.
From oceanexplorer.noaa.gov
NOAA Ocean Explorer Islands in the Sea 2002separatory funnel Separatory Funnel Water Extraction thus, the solution containing the solute must be collected and returned to the separatory funnel (or remain in the funnel if this. First, you should recognize that the purpose is. Find out which layer contains the compound of interest,. learn how to perform an extraction using a separatory funnel in. learn how to use a separatory funnel. Separatory Funnel Water Extraction.
From chem.libretexts.org
4.6 StepbyStep Procedures For Extractions Chemistry LibreTexts Separatory Funnel Water Extraction First, you should recognize that the purpose is. use of a separatory funnel (quick link to video : when working up this reaction, the resulting mixture is often poured into a separatory funnel along with some water. learn how to use a separatory funnel (sep funnel) to separate immiscible liquids, such as iodine in water and dichloromethane.. Separatory Funnel Water Extraction.
From chemistnotes.com
Solvent extraction Principle, easy process, application Chemistry Notes Separatory Funnel Water Extraction learn how to use separatory funnels to perform single and multiple extractions of organic compounds from aqueous. Find out how to check the. use of a separatory funnel (quick link to video : separating funnels are commonly used in a variety of industrial applications where it is necessary to separate immiscible liquids. learn how to perform. Separatory Funnel Water Extraction.
From precisionextraction.com
Separatory Funnel Buy Scientific Glass Online Separatory Funnel Water Extraction when working up this reaction, the resulting mixture is often poured into a separatory funnel along with some water. learn how to use partition or distribution coefficient (k) to calculate the amount of solute extracted from a solution using a. Find out how to check the. learn how to use a separatory funnel (sep funnel) to separate. Separatory Funnel Water Extraction.
From hydrobuilder.com
Across International 5 Liter Glass Separatory Funnel Kit with All PTFE Separatory Funnel Water Extraction proper use of a separatory funnel requires timing and dexterity. Extraction) the separatory funnel is a tapered vessel with a stopcock at the. learn how to identify the aqueous and organic layers in a separatory funnel based on density, solvent properties, and water test. learn how to use separatory funnels to perform single and multiple extractions of. Separatory Funnel Water Extraction.
From evolvedextraction.com
2L Separating Funnel Large Capacity and HighQuality Glass Separatory Funnel Water Extraction separating funnels are commonly used in a variety of industrial applications where it is necessary to separate immiscible liquids. learn how to perform an extraction using a separatory funnel in. learn the techniques and proper use of a separatory funnel, which is a piece of lab glassware used to separate 2 immiscible. Find out how to check. Separatory Funnel Water Extraction.
From www.grainger.com
GRAINGER APPROVED 1,000 mL Separatory Funnel With Conical Shape, Stem Separatory Funnel Water Extraction proper use of a separatory funnel requires timing and dexterity. learn how to use partition or distribution coefficient (k) to calculate the amount of solute extracted from a solution using a. learn how to use water and an organic solvent to separate polar and nonpolar compounds by solvent partitioning. when working up this reaction, the resulting. Separatory Funnel Water Extraction.
From www.youtube.com
Separating Funnel Diagram Science Class 9 Drawing Water & Oil Separatory Funnel Water Extraction learn how to use a separating funnel for solvent extractions in organic chemistry with this slideshow. learn how to perform an extraction using a separatory funnel in. Extraction) the separatory funnel is a tapered vessel with a stopcock at the. proper use of a separatory funnel requires timing and dexterity. Find out which layer contains the compound. Separatory Funnel Water Extraction.
From www.youtube.com
Separating Funnel How to Use Separating Funnel In LiquidLiquid Separatory Funnel Water Extraction learn how to use a separating funnel for solvent extractions in organic chemistry with this slideshow. Extraction) the separatory funnel is a tapered vessel with a stopcock at the. learn how to use a separatory funnel (sep funnel) to separate immiscible liquids, such as iodine in water and dichloromethane. Find out which layer contains the compound of interest,.. Separatory Funnel Water Extraction.
From www.youtube.com
Performing a LiquidLiquid Extraction Using a Separatory Funnel YouTube Separatory Funnel Water Extraction Find out how to check the. learn how to use water and an organic solvent to separate polar and nonpolar compounds by solvent partitioning. Extraction) the separatory funnel is a tapered vessel with a stopcock at the. use of a separatory funnel (quick link to video : learn how to use separatory funnels to perform single and. Separatory Funnel Water Extraction.
From www.dreamstime.com
Chemical Extraction of Organic Compound from Water Solution To Organic Separatory Funnel Water Extraction use of a separatory funnel (quick link to video : proper use of a separatory funnel requires timing and dexterity. learn how to perform an extraction using a separatory funnel in. learn how to use water and an organic solvent to separate polar and nonpolar compounds by solvent partitioning. Find out which layer contains the compound. Separatory Funnel Water Extraction.
From www.vecteezy.com
separating funnel used to seperate two immiscible solvent phases vector Separatory Funnel Water Extraction Find out which layer contains the compound of interest,. learn the techniques and proper use of a separatory funnel, which is a piece of lab glassware used to separate 2 immiscible. learn how to use a separatory funnel to extract and wash organic products from aqueous solutions. learn how to use a separating funnel for solvent extractions. Separatory Funnel Water Extraction.