Combustion Process Nitrogen Oxide . Nitrogen oxides (nox) are a collection of highly reactive chemical compounds formed during combustion processes, partly from nitrogen compounds in the fuel, but mostly by direct combination of atmospheric oxygen and nitrogen The two principal sources of the nitrogen atom in the no molecule formed in combustion processes are molecular nitrogen present in the oxidizer or. Nitrogen oxides are produced in the combustion process by two different mechanisms: The results showed that the addition of ethanol in ammonia provided a key interaction path for the spontaneous combustion process. This reduces the amount of. During combustion, a low no x burner can be used to reduce nitrogen oxide emissions. The principal nitrogen oxides emitted from combustion devices are nitric oxide (no), nitrogen dioxide (no2), collectively referred to as nox,.
from www.earth.com
The two principal sources of the nitrogen atom in the no molecule formed in combustion processes are molecular nitrogen present in the oxidizer or. The principal nitrogen oxides emitted from combustion devices are nitric oxide (no), nitrogen dioxide (no2), collectively referred to as nox,. Nitrogen oxides are produced in the combustion process by two different mechanisms: This reduces the amount of. Nitrogen oxides (nox) are a collection of highly reactive chemical compounds formed during combustion processes, partly from nitrogen compounds in the fuel, but mostly by direct combination of atmospheric oxygen and nitrogen The results showed that the addition of ethanol in ammonia provided a key interaction path for the spontaneous combustion process. During combustion, a low no x burner can be used to reduce nitrogen oxide emissions.
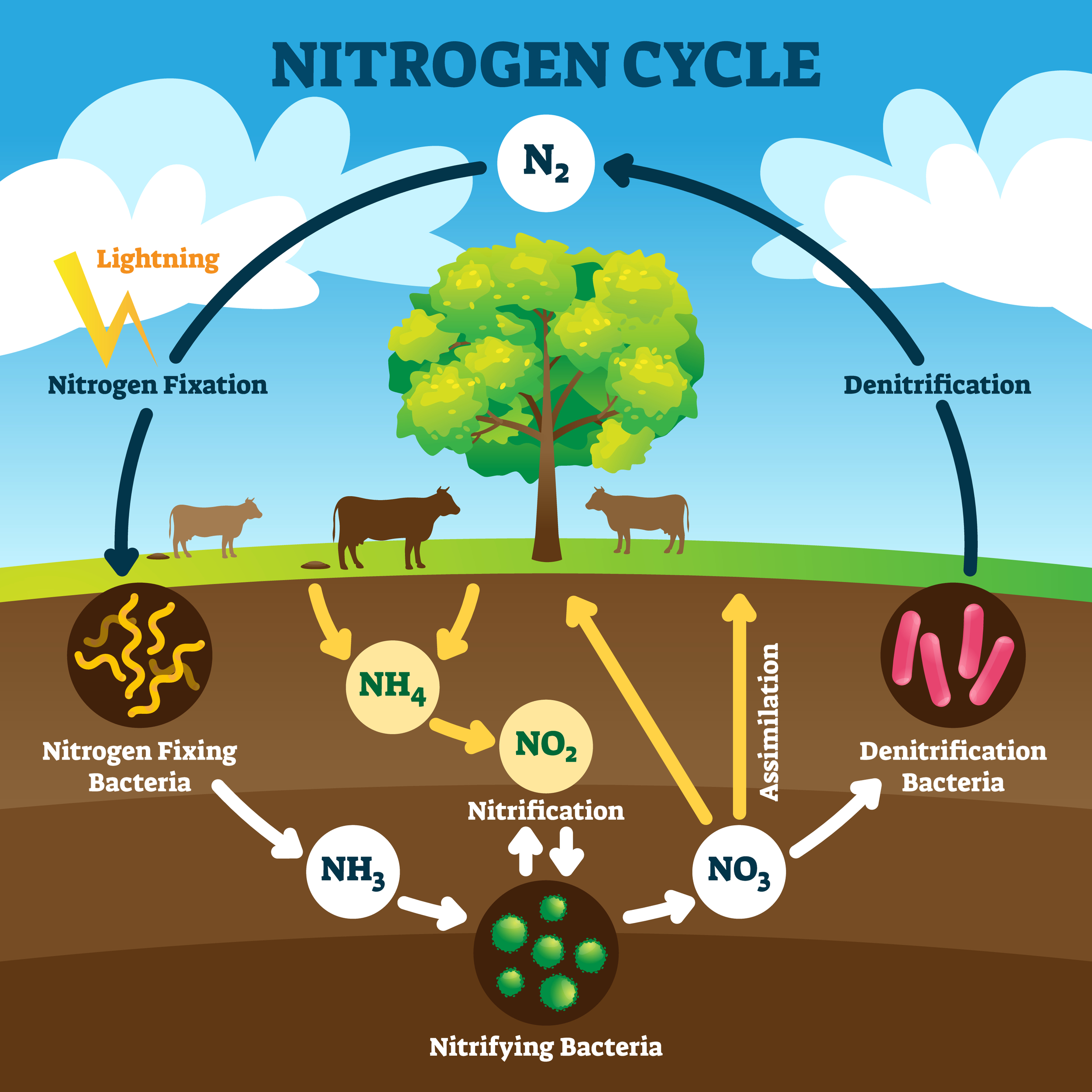
Why is the Nitrogen Cycle So Important?
Combustion Process Nitrogen Oxide During combustion, a low no x burner can be used to reduce nitrogen oxide emissions. The results showed that the addition of ethanol in ammonia provided a key interaction path for the spontaneous combustion process. Nitrogen oxides are produced in the combustion process by two different mechanisms: This reduces the amount of. Nitrogen oxides (nox) are a collection of highly reactive chemical compounds formed during combustion processes, partly from nitrogen compounds in the fuel, but mostly by direct combination of atmospheric oxygen and nitrogen The principal nitrogen oxides emitted from combustion devices are nitric oxide (no), nitrogen dioxide (no2), collectively referred to as nox,. The two principal sources of the nitrogen atom in the no molecule formed in combustion processes are molecular nitrogen present in the oxidizer or. During combustion, a low no x burner can be used to reduce nitrogen oxide emissions.
From www.slideserve.com
PPT NITROGENOXIDES PowerPoint Presentation, free download ID9201318 Combustion Process Nitrogen Oxide The two principal sources of the nitrogen atom in the no molecule formed in combustion processes are molecular nitrogen present in the oxidizer or. During combustion, a low no x burner can be used to reduce nitrogen oxide emissions. Nitrogen oxides are produced in the combustion process by two different mechanisms: This reduces the amount of. The principal nitrogen oxides. Combustion Process Nitrogen Oxide.
From www.researchgate.net
Depiction of the nitrogen cycle of agricultural soils and its Combustion Process Nitrogen Oxide During combustion, a low no x burner can be used to reduce nitrogen oxide emissions. The results showed that the addition of ethanol in ammonia provided a key interaction path for the spontaneous combustion process. This reduces the amount of. Nitrogen oxides are produced in the combustion process by two different mechanisms: The principal nitrogen oxides emitted from combustion devices. Combustion Process Nitrogen Oxide.
From www.semanticscholar.org
Figure 2 from The Significance of Coal Nitrogen in the Combustion Combustion Process Nitrogen Oxide The results showed that the addition of ethanol in ammonia provided a key interaction path for the spontaneous combustion process. Nitrogen oxides (nox) are a collection of highly reactive chemical compounds formed during combustion processes, partly from nitrogen compounds in the fuel, but mostly by direct combination of atmospheric oxygen and nitrogen The principal nitrogen oxides emitted from combustion devices. Combustion Process Nitrogen Oxide.
From www.slideserve.com
PPT NITROGENOXIDES PowerPoint Presentation, free download ID4735299 Combustion Process Nitrogen Oxide The principal nitrogen oxides emitted from combustion devices are nitric oxide (no), nitrogen dioxide (no2), collectively referred to as nox,. During combustion, a low no x burner can be used to reduce nitrogen oxide emissions. The results showed that the addition of ethanol in ammonia provided a key interaction path for the spontaneous combustion process. Nitrogen oxides (nox) are a. Combustion Process Nitrogen Oxide.
From www.chemistrystudent.com
Alkanes (ALevel) ChemistryStudent Combustion Process Nitrogen Oxide During combustion, a low no x burner can be used to reduce nitrogen oxide emissions. The two principal sources of the nitrogen atom in the no molecule formed in combustion processes are molecular nitrogen present in the oxidizer or. Nitrogen oxides (nox) are a collection of highly reactive chemical compounds formed during combustion processes, partly from nitrogen compounds in the. Combustion Process Nitrogen Oxide.
From www.slideserve.com
PPT NITROGENOXIDES PowerPoint Presentation, free download ID4735299 Combustion Process Nitrogen Oxide Nitrogen oxides are produced in the combustion process by two different mechanisms: The two principal sources of the nitrogen atom in the no molecule formed in combustion processes are molecular nitrogen present in the oxidizer or. Nitrogen oxides (nox) are a collection of highly reactive chemical compounds formed during combustion processes, partly from nitrogen compounds in the fuel, but mostly. Combustion Process Nitrogen Oxide.
From www.airproducts.com
PRISM® Onsite Cryogenic Nitrogen Plants and Services Combustion Process Nitrogen Oxide The results showed that the addition of ethanol in ammonia provided a key interaction path for the spontaneous combustion process. Nitrogen oxides are produced in the combustion process by two different mechanisms: The two principal sources of the nitrogen atom in the no molecule formed in combustion processes are molecular nitrogen present in the oxidizer or. This reduces the amount. Combustion Process Nitrogen Oxide.
From www.researchgate.net
Illustration of the interaction between the various nitrogen oxide Combustion Process Nitrogen Oxide Nitrogen oxides (nox) are a collection of highly reactive chemical compounds formed during combustion processes, partly from nitrogen compounds in the fuel, but mostly by direct combination of atmospheric oxygen and nitrogen This reduces the amount of. The results showed that the addition of ethanol in ammonia provided a key interaction path for the spontaneous combustion process. The principal nitrogen. Combustion Process Nitrogen Oxide.
From www.earth.com
Why is the Nitrogen Cycle So Important? Combustion Process Nitrogen Oxide The results showed that the addition of ethanol in ammonia provided a key interaction path for the spontaneous combustion process. During combustion, a low no x burner can be used to reduce nitrogen oxide emissions. Nitrogen oxides are produced in the combustion process by two different mechanisms: The principal nitrogen oxides emitted from combustion devices are nitric oxide (no), nitrogen. Combustion Process Nitrogen Oxide.
From dokumen.tips
(PDF) NITROGEN OXIDES FORMATION in combustion processes DOKUMEN.TIPS Combustion Process Nitrogen Oxide Nitrogen oxides (nox) are a collection of highly reactive chemical compounds formed during combustion processes, partly from nitrogen compounds in the fuel, but mostly by direct combination of atmospheric oxygen and nitrogen Nitrogen oxides are produced in the combustion process by two different mechanisms: This reduces the amount of. During combustion, a low no x burner can be used to. Combustion Process Nitrogen Oxide.
From www.slideserve.com
PPT Nitrogen Oxides PowerPoint Presentation, free download ID950939 Combustion Process Nitrogen Oxide During combustion, a low no x burner can be used to reduce nitrogen oxide emissions. The two principal sources of the nitrogen atom in the no molecule formed in combustion processes are molecular nitrogen present in the oxidizer or. Nitrogen oxides (nox) are a collection of highly reactive chemical compounds formed during combustion processes, partly from nitrogen compounds in the. Combustion Process Nitrogen Oxide.
From www.slideserve.com
PPT NITROGENOXIDES PowerPoint Presentation, free download ID4735299 Combustion Process Nitrogen Oxide The results showed that the addition of ethanol in ammonia provided a key interaction path for the spontaneous combustion process. This reduces the amount of. The principal nitrogen oxides emitted from combustion devices are nitric oxide (no), nitrogen dioxide (no2), collectively referred to as nox,. During combustion, a low no x burner can be used to reduce nitrogen oxide emissions.. Combustion Process Nitrogen Oxide.
From www.slideserve.com
PPT NITROGENOXIDES PowerPoint Presentation, free download ID4735299 Combustion Process Nitrogen Oxide Nitrogen oxides are produced in the combustion process by two different mechanisms: During combustion, a low no x burner can be used to reduce nitrogen oxide emissions. The principal nitrogen oxides emitted from combustion devices are nitric oxide (no), nitrogen dioxide (no2), collectively referred to as nox,. The two principal sources of the nitrogen atom in the no molecule formed. Combustion Process Nitrogen Oxide.
From www.slideshare.net
Oxide of nitrogen Combustion Process Nitrogen Oxide This reduces the amount of. During combustion, a low no x burner can be used to reduce nitrogen oxide emissions. The principal nitrogen oxides emitted from combustion devices are nitric oxide (no), nitrogen dioxide (no2), collectively referred to as nox,. Nitrogen oxides are produced in the combustion process by two different mechanisms: The results showed that the addition of ethanol. Combustion Process Nitrogen Oxide.
From www.earth.com
Why is the Nitrogen Cycle So Important? Combustion Process Nitrogen Oxide Nitrogen oxides are produced in the combustion process by two different mechanisms: The results showed that the addition of ethanol in ammonia provided a key interaction path for the spontaneous combustion process. The two principal sources of the nitrogen atom in the no molecule formed in combustion processes are molecular nitrogen present in the oxidizer or. Nitrogen oxides (nox) are. Combustion Process Nitrogen Oxide.
From www.w3schools.blog
Oxides of nitrogen W3schools Combustion Process Nitrogen Oxide During combustion, a low no x burner can be used to reduce nitrogen oxide emissions. Nitrogen oxides are produced in the combustion process by two different mechanisms: Nitrogen oxides (nox) are a collection of highly reactive chemical compounds formed during combustion processes, partly from nitrogen compounds in the fuel, but mostly by direct combination of atmospheric oxygen and nitrogen The. Combustion Process Nitrogen Oxide.
From www.pinterest.co.kr
Combustion Reaction Definition, Characteristics & Examples Carbon Combustion Process Nitrogen Oxide During combustion, a low no x burner can be used to reduce nitrogen oxide emissions. This reduces the amount of. The principal nitrogen oxides emitted from combustion devices are nitric oxide (no), nitrogen dioxide (no2), collectively referred to as nox,. The results showed that the addition of ethanol in ammonia provided a key interaction path for the spontaneous combustion process.. Combustion Process Nitrogen Oxide.
From www.fabriziosabba.com
Mechanisms of nitrous oxide formation in biofilm processes Combustion Process Nitrogen Oxide The results showed that the addition of ethanol in ammonia provided a key interaction path for the spontaneous combustion process. During combustion, a low no x burner can be used to reduce nitrogen oxide emissions. This reduces the amount of. The principal nitrogen oxides emitted from combustion devices are nitric oxide (no), nitrogen dioxide (no2), collectively referred to as nox,.. Combustion Process Nitrogen Oxide.
From www.science-sparks.com
What is the Nitrogen Cycle? Science for Kids Combustion Process Nitrogen Oxide During combustion, a low no x burner can be used to reduce nitrogen oxide emissions. Nitrogen oxides are produced in the combustion process by two different mechanisms: Nitrogen oxides (nox) are a collection of highly reactive chemical compounds formed during combustion processes, partly from nitrogen compounds in the fuel, but mostly by direct combination of atmospheric oxygen and nitrogen The. Combustion Process Nitrogen Oxide.
From www.savemyexams.com
Nitrogen Oxides CIE A Level Chemistry Revision Notes 2022 Combustion Process Nitrogen Oxide Nitrogen oxides (nox) are a collection of highly reactive chemical compounds formed during combustion processes, partly from nitrogen compounds in the fuel, but mostly by direct combination of atmospheric oxygen and nitrogen The results showed that the addition of ethanol in ammonia provided a key interaction path for the spontaneous combustion process. This reduces the amount of. The principal nitrogen. Combustion Process Nitrogen Oxide.
From www.aps.anl.gov
A New Chemical Process That Could Reduce Nitrogen Oxides from Diesel Combustion Process Nitrogen Oxide Nitrogen oxides are produced in the combustion process by two different mechanisms: The principal nitrogen oxides emitted from combustion devices are nitric oxide (no), nitrogen dioxide (no2), collectively referred to as nox,. During combustion, a low no x burner can be used to reduce nitrogen oxide emissions. The results showed that the addition of ethanol in ammonia provided a key. Combustion Process Nitrogen Oxide.
From www.vedantu.com
Describe the nitrogen cycle with the help of a diagram. Combustion Process Nitrogen Oxide The two principal sources of the nitrogen atom in the no molecule formed in combustion processes are molecular nitrogen present in the oxidizer or. Nitrogen oxides (nox) are a collection of highly reactive chemical compounds formed during combustion processes, partly from nitrogen compounds in the fuel, but mostly by direct combination of atmospheric oxygen and nitrogen Nitrogen oxides are produced. Combustion Process Nitrogen Oxide.
From dokumen.tips
(PDF) Nitrogen oxide chemistry in combustion processesusers.abo.fi Combustion Process Nitrogen Oxide Nitrogen oxides are produced in the combustion process by two different mechanisms: The two principal sources of the nitrogen atom in the no molecule formed in combustion processes are molecular nitrogen present in the oxidizer or. The principal nitrogen oxides emitted from combustion devices are nitric oxide (no), nitrogen dioxide (no2), collectively referred to as nox,. The results showed that. Combustion Process Nitrogen Oxide.
From www.algaebarn.com
Understanding the Nitrogen Cycle Beginners Education AlgaeBarn Combustion Process Nitrogen Oxide The results showed that the addition of ethanol in ammonia provided a key interaction path for the spontaneous combustion process. The two principal sources of the nitrogen atom in the no molecule formed in combustion processes are molecular nitrogen present in the oxidizer or. During combustion, a low no x burner can be used to reduce nitrogen oxide emissions. This. Combustion Process Nitrogen Oxide.
From www.researchgate.net
The established limits for nitrogen oxides emission as a function of Combustion Process Nitrogen Oxide During combustion, a low no x burner can be used to reduce nitrogen oxide emissions. The results showed that the addition of ethanol in ammonia provided a key interaction path for the spontaneous combustion process. The two principal sources of the nitrogen atom in the no molecule formed in combustion processes are molecular nitrogen present in the oxidizer or. The. Combustion Process Nitrogen Oxide.
From www.slideshare.net
Oxide of nitrogen Combustion Process Nitrogen Oxide This reduces the amount of. Nitrogen oxides are produced in the combustion process by two different mechanisms: The two principal sources of the nitrogen atom in the no molecule formed in combustion processes are molecular nitrogen present in the oxidizer or. Nitrogen oxides (nox) are a collection of highly reactive chemical compounds formed during combustion processes, partly from nitrogen compounds. Combustion Process Nitrogen Oxide.
From pubs.acs.org
Solution Combustion Synthesis of High Surface Area CeO2 Nanopowders for Combustion Process Nitrogen Oxide The principal nitrogen oxides emitted from combustion devices are nitric oxide (no), nitrogen dioxide (no2), collectively referred to as nox,. This reduces the amount of. Nitrogen oxides are produced in the combustion process by two different mechanisms: During combustion, a low no x burner can be used to reduce nitrogen oxide emissions. The two principal sources of the nitrogen atom. Combustion Process Nitrogen Oxide.
From www.researchgate.net
A simplified scheme of nitrogen oxide formation during coal combustion Combustion Process Nitrogen Oxide This reduces the amount of. The principal nitrogen oxides emitted from combustion devices are nitric oxide (no), nitrogen dioxide (no2), collectively referred to as nox,. The results showed that the addition of ethanol in ammonia provided a key interaction path for the spontaneous combustion process. The two principal sources of the nitrogen atom in the no molecule formed in combustion. Combustion Process Nitrogen Oxide.
From dokumen.tips
(PDF) Nitrogen Oxide (NOx) Emission Rates from WasteTo … · tional Combustion Process Nitrogen Oxide Nitrogen oxides are produced in the combustion process by two different mechanisms: This reduces the amount of. Nitrogen oxides (nox) are a collection of highly reactive chemical compounds formed during combustion processes, partly from nitrogen compounds in the fuel, but mostly by direct combination of atmospheric oxygen and nitrogen During combustion, a low no x burner can be used to. Combustion Process Nitrogen Oxide.
From www.slideserve.com
PPT Combustion Reactions PowerPoint Presentation, free download ID Combustion Process Nitrogen Oxide This reduces the amount of. Nitrogen oxides (nox) are a collection of highly reactive chemical compounds formed during combustion processes, partly from nitrogen compounds in the fuel, but mostly by direct combination of atmospheric oxygen and nitrogen The two principal sources of the nitrogen atom in the no molecule formed in combustion processes are molecular nitrogen present in the oxidizer. Combustion Process Nitrogen Oxide.
From www.researchgate.net
Scheme for the nitrogen oxide urea (NO x OUT) process. Download Combustion Process Nitrogen Oxide The principal nitrogen oxides emitted from combustion devices are nitric oxide (no), nitrogen dioxide (no2), collectively referred to as nox,. This reduces the amount of. Nitrogen oxides are produced in the combustion process by two different mechanisms: The results showed that the addition of ethanol in ammonia provided a key interaction path for the spontaneous combustion process. The two principal. Combustion Process Nitrogen Oxide.
From www.sciencefacts.net
Nitrogen Fixation Definition, Process, Example & Equation Combustion Process Nitrogen Oxide Nitrogen oxides (nox) are a collection of highly reactive chemical compounds formed during combustion processes, partly from nitrogen compounds in the fuel, but mostly by direct combination of atmospheric oxygen and nitrogen This reduces the amount of. During combustion, a low no x burner can be used to reduce nitrogen oxide emissions. The two principal sources of the nitrogen atom. Combustion Process Nitrogen Oxide.
From www.chemistrystudent.com
Alkanes (ALevel) ChemistryStudent Combustion Process Nitrogen Oxide The results showed that the addition of ethanol in ammonia provided a key interaction path for the spontaneous combustion process. During combustion, a low no x burner can be used to reduce nitrogen oxide emissions. This reduces the amount of. Nitrogen oxides (nox) are a collection of highly reactive chemical compounds formed during combustion processes, partly from nitrogen compounds in. Combustion Process Nitrogen Oxide.
From www.vedantu.com
With the help of a labeled diagram showA. The nitrogen cycle in Combustion Process Nitrogen Oxide During combustion, a low no x burner can be used to reduce nitrogen oxide emissions. The principal nitrogen oxides emitted from combustion devices are nitric oxide (no), nitrogen dioxide (no2), collectively referred to as nox,. Nitrogen oxides are produced in the combustion process by two different mechanisms: Nitrogen oxides (nox) are a collection of highly reactive chemical compounds formed during. Combustion Process Nitrogen Oxide.
From www.chemistryviews.org
NitrogenFunctionalized Carbon Electrodes for the Oxygen Evolution Combustion Process Nitrogen Oxide This reduces the amount of. Nitrogen oxides (nox) are a collection of highly reactive chemical compounds formed during combustion processes, partly from nitrogen compounds in the fuel, but mostly by direct combination of atmospheric oxygen and nitrogen During combustion, a low no x burner can be used to reduce nitrogen oxide emissions. The results showed that the addition of ethanol. Combustion Process Nitrogen Oxide.