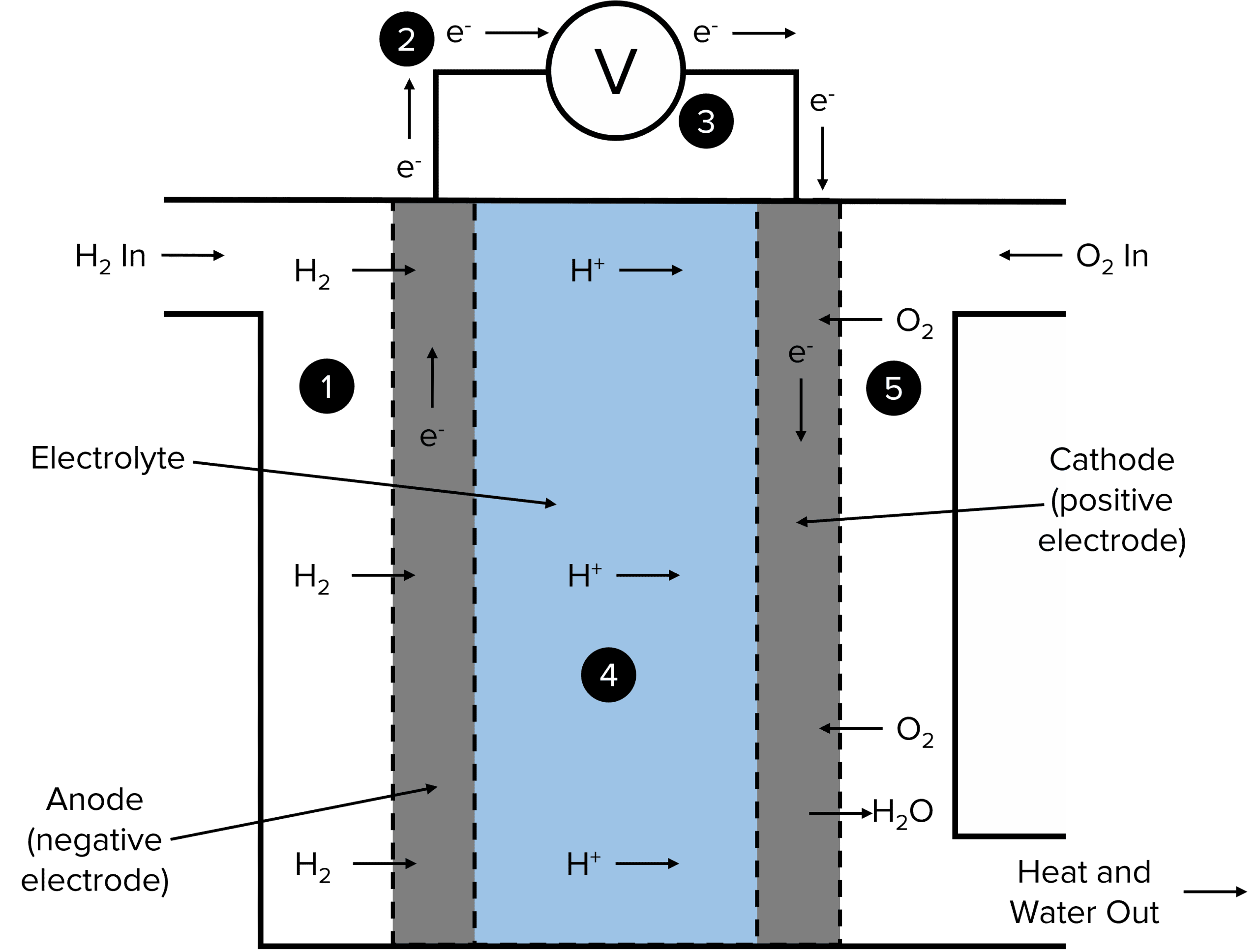
Fuel Cell In Order To Produce Electricity Burns . A fuel cell resembles a battery in. It is very similar to a battery,. A fuel cell consists of two electrodes—a. Fuel cells have an important advantage. Fuel cells work like batteries, but they do not run down or need recharging. A fuel cell uses the chemical energy of hydrogen or other fuels to cleanly and efficiently produce electricity. If hydrogen is the fuel, the only. They produce electricity and heat as long as fuel is supplied. A fuel cell in order to produce electricity burns hydrogen. Fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical reactions. A fuel cell is nothing but a reaction in which. Fuel cells require a continuous input of fuel and an oxidizing agent (generally oxygen) in order to sustain the reactions that generate the electricity. A fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available.
from mmerevise.co.uk
If hydrogen is the fuel, the only. Fuel cells have an important advantage. A fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. They produce electricity and heat as long as fuel is supplied. It is very similar to a battery,. A fuel cell in order to produce electricity burns hydrogen. Fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical reactions. Fuel cells require a continuous input of fuel and an oxidizing agent (generally oxygen) in order to sustain the reactions that generate the electricity. A fuel cell is nothing but a reaction in which. A fuel cell resembles a battery in.
Electrochemical Cells Worksheets and Revision MME
Fuel Cell In Order To Produce Electricity Burns A fuel cell in order to produce electricity burns hydrogen. Fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical reactions. A fuel cell uses the chemical energy of hydrogen or other fuels to cleanly and efficiently produce electricity. Fuel cells require a continuous input of fuel and an oxidizing agent (generally oxygen) in order to sustain the reactions that generate the electricity. A fuel cell consists of two electrodes—a. If hydrogen is the fuel, the only. Fuel cells have an important advantage. Fuel cells work like batteries, but they do not run down or need recharging. It is very similar to a battery,. A fuel cell in order to produce electricity burns hydrogen. A fuel cell resembles a battery in. A fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. They produce electricity and heat as long as fuel is supplied. A fuel cell is nothing but a reaction in which.
From www.researchgate.net
Schematic diagram of the fuel cell in breath analyzers. Download Fuel Cell In Order To Produce Electricity Burns A fuel cell resembles a battery in. Fuel cells have an important advantage. A fuel cell in order to produce electricity burns hydrogen. A fuel cell is nothing but a reaction in which. It is very similar to a battery,. Fuel cells work like batteries, but they do not run down or need recharging. A fuel cell consists of two. Fuel Cell In Order To Produce Electricity Burns.
From www.jove.com
In this hydrogen fuel cell, oxygen reacts with hydrogen, producing Fuel Cell In Order To Produce Electricity Burns Fuel cells work like batteries, but they do not run down or need recharging. A fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. Fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical reactions.. Fuel Cell In Order To Produce Electricity Burns.
From www.slideserve.com
PPT Fuel cells and hydrogen storage PowerPoint Presentation, free Fuel Cell In Order To Produce Electricity Burns A fuel cell uses the chemical energy of hydrogen or other fuels to cleanly and efficiently produce electricity. A fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. A fuel cell resembles a battery in. A fuel cell is nothing but a reaction in which. Fuel. Fuel Cell In Order To Produce Electricity Burns.
From www.chfca.ca
About Fuel Cells CHFCA Fuel Cell In Order To Produce Electricity Burns A fuel cell is nothing but a reaction in which. A fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. Fuel cells work like batteries, but they do not run down or need recharging. If hydrogen is the fuel, the only. Fuel cells require a continuous. Fuel Cell In Order To Produce Electricity Burns.
From www.electroniclinic.com
Hydrogen Fuel Cell, Application of Fuel Cells, construction, and Working Fuel Cell In Order To Produce Electricity Burns A fuel cell uses the chemical energy of hydrogen or other fuels to cleanly and efficiently produce electricity. If hydrogen is the fuel, the only. Fuel cells have an important advantage. A fuel cell is nothing but a reaction in which. Fuel cells require a continuous input of fuel and an oxidizing agent (generally oxygen) in order to sustain the. Fuel Cell In Order To Produce Electricity Burns.
From climatebiz.com
Hydrogen fuel cells vs. lithiumion batteries Powering EVs Fuel Cell In Order To Produce Electricity Burns They produce electricity and heat as long as fuel is supplied. A fuel cell uses the chemical energy of hydrogen or other fuels to cleanly and efficiently produce electricity. A fuel cell in order to produce electricity burns hydrogen. A fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and. Fuel Cell In Order To Produce Electricity Burns.
From www.slideserve.com
PPT Fuel Cell Technology PowerPoint Presentation, free download ID Fuel Cell In Order To Produce Electricity Burns A fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. Fuel cells work like batteries, but they do not run down or need recharging. They produce electricity and heat as long as fuel is supplied. Fuel cells have an important advantage. Fuel cell, any of a. Fuel Cell In Order To Produce Electricity Burns.
From chemwiki.ucdavis.edu
Fuel Cell Stack Diagram IK.png Fuel Cell In Order To Produce Electricity Burns It is very similar to a battery,. A fuel cell uses the chemical energy of hydrogen or other fuels to cleanly and efficiently produce electricity. They produce electricity and heat as long as fuel is supplied. A fuel cell consists of two electrodes—a. Fuel cell, any of a class of devices that convert the chemical energy of a fuel directly. Fuel Cell In Order To Produce Electricity Burns.
From electraschematics.com
The Complete Guide to Wiring a Fuel Cell Sending Unit Fuel Cell In Order To Produce Electricity Burns Fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical reactions. Fuel cells work like batteries, but they do not run down or need recharging. If hydrogen is the fuel, the only. Fuel cells have an important advantage. A fuel cell uses the chemical energy of hydrogen or other. Fuel Cell In Order To Produce Electricity Burns.
From www.mdpi.com
Energies Free FullText Future of Electric and Hydrogen Cars and Fuel Cell In Order To Produce Electricity Burns Fuel cells have an important advantage. Fuel cells work like batteries, but they do not run down or need recharging. A fuel cell uses the chemical energy of hydrogen or other fuels to cleanly and efficiently produce electricity. A fuel cell is nothing but a reaction in which. Fuel cells require a continuous input of fuel and an oxidizing agent. Fuel Cell In Order To Produce Electricity Burns.
From www.fuelcellenergy.com
Benefits of Fuel Cells in Energy Efficient Power Generation Fuel Cell In Order To Produce Electricity Burns A fuel cell uses the chemical energy of hydrogen or other fuels to cleanly and efficiently produce electricity. A fuel cell consists of two electrodes—a. Fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical reactions. A fuel cell is nothing but a reaction in which. If hydrogen is. Fuel Cell In Order To Produce Electricity Burns.
From www.slideserve.com
PPT Fuel Cells PowerPoint Presentation, free download ID513173 Fuel Cell In Order To Produce Electricity Burns It is very similar to a battery,. A fuel cell is nothing but a reaction in which. A fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. Fuel cells work like batteries, but they do not run down or need recharging. A fuel cell resembles a. Fuel Cell In Order To Produce Electricity Burns.
From www.pinterest.com
Alkaline Fuel Cell Advantages Limitations Applications Fuel cell Fuel Cell In Order To Produce Electricity Burns Fuel cells require a continuous input of fuel and an oxidizing agent (generally oxygen) in order to sustain the reactions that generate the electricity. Fuel cells work like batteries, but they do not run down or need recharging. Fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical reactions.. Fuel Cell In Order To Produce Electricity Burns.
From mmerevise.co.uk
Electrochemical Cells Worksheets and Revision MME Fuel Cell In Order To Produce Electricity Burns A fuel cell in order to produce electricity burns hydrogen. A fuel cell resembles a battery in. A fuel cell is nothing but a reaction in which. A fuel cell consists of two electrodes—a. Fuel cells have an important advantage. If hydrogen is the fuel, the only. It is very similar to a battery,. Fuel cells work like batteries, but. Fuel Cell In Order To Produce Electricity Burns.
From www.change.org
Petition · The Need of Focus on Fuel Cells · Fuel Cell In Order To Produce Electricity Burns A fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. A fuel cell is nothing but a reaction in which. A fuel cell resembles a battery in. A fuel cell in order to produce electricity burns hydrogen. If hydrogen is the fuel, the only. They produce. Fuel Cell In Order To Produce Electricity Burns.
From www.mdpi.com
Energies Free FullText Optimal Parameter Identification of a PEM Fuel Cell In Order To Produce Electricity Burns A fuel cell uses the chemical energy of hydrogen or other fuels to cleanly and efficiently produce electricity. Fuel cells have an important advantage. A fuel cell consists of two electrodes—a. A fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. A fuel cell is nothing. Fuel Cell In Order To Produce Electricity Burns.
From www.engineeringity.com
Breaking Down Fuel Cells How they Work and their Components The Fuel Cell In Order To Produce Electricity Burns If hydrogen is the fuel, the only. A fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. It is very similar to a battery,. A fuel cell uses the chemical energy of hydrogen or other fuels to cleanly and efficiently produce electricity. Fuel cells require a. Fuel Cell In Order To Produce Electricity Burns.
From www.researchgate.net
Schematic of an individual fuel cell. In this general case, the Fuel Cell In Order To Produce Electricity Burns Fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical reactions. A fuel cell resembles a battery in. They produce electricity and heat as long as fuel is supplied. A fuel cell is nothing but a reaction in which. It is very similar to a battery,. A fuel cell. Fuel Cell In Order To Produce Electricity Burns.
From www.researchgate.net
Internal schematic of a fuel cell. Download Scientific Diagram Fuel Cell In Order To Produce Electricity Burns A fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. Fuel cells work like batteries, but they do not run down or need recharging. A fuel cell resembles a battery in. It is very similar to a battery,. Fuel cell, any of a class of devices. Fuel Cell In Order To Produce Electricity Burns.
From www.slideserve.com
PPT Fuel Cell Technology PowerPoint Presentation, free download ID Fuel Cell In Order To Produce Electricity Burns If hydrogen is the fuel, the only. A fuel cell consists of two electrodes—a. A fuel cell is nothing but a reaction in which. It is very similar to a battery,. A fuel cell uses the chemical energy of hydrogen or other fuels to cleanly and efficiently produce electricity. A fuel cell like this will continue to operate and produce. Fuel Cell In Order To Produce Electricity Burns.
From fuelcellscars.com
ALL ABOUT FUEL CELLS HOW DO THEY WORK Fuel Cell In Order To Produce Electricity Burns Fuel cells work like batteries, but they do not run down or need recharging. Fuel cells have an important advantage. A fuel cell in order to produce electricity burns hydrogen. Fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical reactions. They produce electricity and heat as long as. Fuel Cell In Order To Produce Electricity Burns.
From www.elomatic.com
Fuel cells Elomatic Fuel Cell In Order To Produce Electricity Burns A fuel cell consists of two electrodes—a. A fuel cell in order to produce electricity burns hydrogen. Fuel cells work like batteries, but they do not run down or need recharging. A fuel cell uses the chemical energy of hydrogen or other fuels to cleanly and efficiently produce electricity. Fuel cells have an important advantage. A fuel cell resembles a. Fuel Cell In Order To Produce Electricity Burns.
From www.britannica.com
Fuel cell Definition, Types, Applications, & Facts Britannica Fuel Cell In Order To Produce Electricity Burns Fuel cells work like batteries, but they do not run down or need recharging. They produce electricity and heat as long as fuel is supplied. A fuel cell consists of two electrodes—a. Fuel cells require a continuous input of fuel and an oxidizing agent (generally oxygen) in order to sustain the reactions that generate the electricity. A fuel cell like. Fuel Cell In Order To Produce Electricity Burns.
From www.researchgate.net
A schematic diagram showing a hydrogen based fuel cell [7]. Download Fuel Cell In Order To Produce Electricity Burns A fuel cell uses the chemical energy of hydrogen or other fuels to cleanly and efficiently produce electricity. Fuel cells work like batteries, but they do not run down or need recharging. They produce electricity and heat as long as fuel is supplied. Fuel cells have an important advantage. Fuel cell, any of a class of devices that convert the. Fuel Cell In Order To Produce Electricity Burns.
From www.biologic.net
What are PEM Fuel Cells and Electrolyzers? BioLogic Learning Center Fuel Cell In Order To Produce Electricity Burns Fuel cells have an important advantage. Fuel cells work like batteries, but they do not run down or need recharging. If hydrogen is the fuel, the only. A fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. A fuel cell is nothing but a reaction in. Fuel Cell In Order To Produce Electricity Burns.
From www.myelectrical2015.com
Electrical Revolution Fuel Cell In Order To Produce Electricity Burns Fuel cells work like batteries, but they do not run down or need recharging. A fuel cell in order to produce electricity burns hydrogen. If hydrogen is the fuel, the only. They produce electricity and heat as long as fuel is supplied. A fuel cell is nothing but a reaction in which. A fuel cell resembles a battery in. A. Fuel Cell In Order To Produce Electricity Burns.
From cafcp.org
Frequently Asked Questions California Fuel Cell Partnership Fuel Cell In Order To Produce Electricity Burns Fuel cells work like batteries, but they do not run down or need recharging. They produce electricity and heat as long as fuel is supplied. Fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical reactions. A fuel cell consists of two electrodes—a. A fuel cell resembles a battery. Fuel Cell In Order To Produce Electricity Burns.
From nl.mathworks.com
Fuel Cell Model MATLAB & Simulink Fuel Cell In Order To Produce Electricity Burns A fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. Fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical reactions. It is very similar to a battery,. A fuel cell in order to produce. Fuel Cell In Order To Produce Electricity Burns.
From energyeducation.ca
Fuel cell Energy Education Fuel Cell In Order To Produce Electricity Burns Fuel cells require a continuous input of fuel and an oxidizing agent (generally oxygen) in order to sustain the reactions that generate the electricity. A fuel cell uses the chemical energy of hydrogen or other fuels to cleanly and efficiently produce electricity. Fuel cells work like batteries, but they do not run down or need recharging. Fuel cells have an. Fuel Cell In Order To Produce Electricity Burns.
From boffinsportal.com
8 Conversion of Chemical Energy to Electrical Energy Examples The Fuel Cell In Order To Produce Electricity Burns If hydrogen is the fuel, the only. Fuel cells work like batteries, but they do not run down or need recharging. Fuel cells have an important advantage. They produce electricity and heat as long as fuel is supplied. Fuel cells require a continuous input of fuel and an oxidizing agent (generally oxygen) in order to sustain the reactions that generate. Fuel Cell In Order To Produce Electricity Burns.
From powerup-tech.com
The ABC of Fuel Cells PowerUP Energy Technologies Fuel Cell In Order To Produce Electricity Burns A fuel cell consists of two electrodes—a. A fuel cell resembles a battery in. A fuel cell in order to produce electricity burns hydrogen. If hydrogen is the fuel, the only. A fuel cell is nothing but a reaction in which. Fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity. Fuel Cell In Order To Produce Electricity Burns.
From www.myelectrical2015.com
Electrical Revolution Working Principle of Fuel Cell Fuel Cell In Order To Produce Electricity Burns Fuel cells require a continuous input of fuel and an oxidizing agent (generally oxygen) in order to sustain the reactions that generate the electricity. A fuel cell is nothing but a reaction in which. They produce electricity and heat as long as fuel is supplied. Fuel cells work like batteries, but they do not run down or need recharging. A. Fuel Cell In Order To Produce Electricity Burns.
From www.drugdeliverybusiness.com
This implantable fuel cell generates electricity from glucose Fuel Cell In Order To Produce Electricity Burns They produce electricity and heat as long as fuel is supplied. Fuel cells have an important advantage. A fuel cell uses the chemical energy of hydrogen or other fuels to cleanly and efficiently produce electricity. Fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical reactions. Fuel cells work. Fuel Cell In Order To Produce Electricity Burns.
From kindle-tech.com
Understanding Electrolytic Cells Conversion Of Energy And Applications Fuel Cell In Order To Produce Electricity Burns A fuel cell is nothing but a reaction in which. They produce electricity and heat as long as fuel is supplied. Fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical reactions. A fuel cell in order to produce electricity burns hydrogen. Fuel cells require a continuous input of. Fuel Cell In Order To Produce Electricity Burns.
From www.powerelectronicsnews.com
PEM Fuel Cell Modeling and Simulation Power Electronics News Fuel Cell In Order To Produce Electricity Burns A fuel cell resembles a battery in. A fuel cell consists of two electrodes—a. Fuel cells require a continuous input of fuel and an oxidizing agent (generally oxygen) in order to sustain the reactions that generate the electricity. A fuel cell is nothing but a reaction in which. A fuel cell uses the chemical energy of hydrogen or other fuels. Fuel Cell In Order To Produce Electricity Burns.