Joules Lead Heat Capacity . Different substances have different specific heat capacities. For example, the heat required to raise the temperature of 1 kg of water by 1 k is 4184 joules, so the specific. 71 rows specific heat capacity of metals table chart. Specific heat, or specific heat capacity, is a property related to internal energy that is very important in. How many joules of heat are required to heat 69 g of lead from 25.5 degree celsius to 85.6 degree celsius? The specific heat is the amount of heat energy per unit mass required to raise the temperature by. The specific heat capacity of lead. 16 rows specific heats and molar heat capacities for various substances at 20 c substance c in j/gm k c in cal/gm k or btu/lb f molar c j/mol k. The si unit of specific heat capacity is joule per kelvin per kilogram, j⋅kg −1 ⋅k −1. Specific heat of lead is 0.13 j/g k. 55 rows the table of specific heat capacities gives the volumetric heat capacity as well as the specific heat capacity of some substances and. For example, the specific heat capacity of water is 4,180 j/kg°c, but the.
from studylib.net
71 rows specific heat capacity of metals table chart. For example, the heat required to raise the temperature of 1 kg of water by 1 k is 4184 joules, so the specific. The si unit of specific heat capacity is joule per kelvin per kilogram, j⋅kg −1 ⋅k −1. Specific heat of lead is 0.13 j/g k. For example, the specific heat capacity of water is 4,180 j/kg°c, but the. How many joules of heat are required to heat 69 g of lead from 25.5 degree celsius to 85.6 degree celsius? The specific heat is the amount of heat energy per unit mass required to raise the temperature by. Specific heat, or specific heat capacity, is a property related to internal energy that is very important in. 16 rows specific heats and molar heat capacities for various substances at 20 c substance c in j/gm k c in cal/gm k or btu/lb f molar c j/mol k. Different substances have different specific heat capacities.
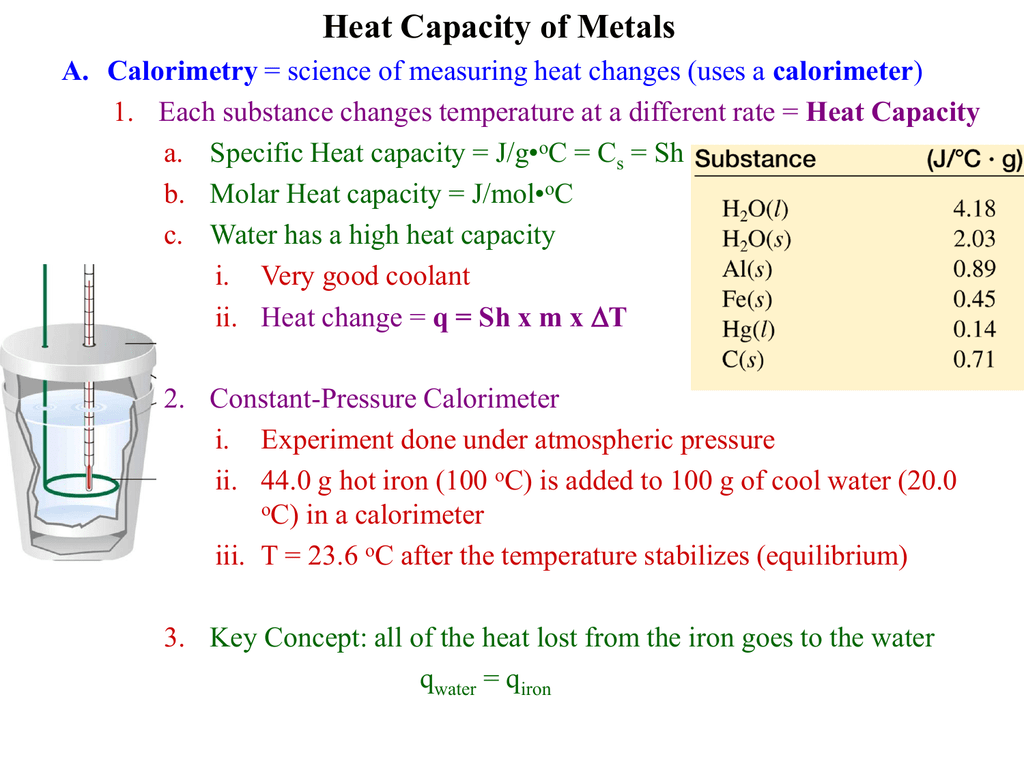
Heat Capacity of Metals PreLab
Joules Lead Heat Capacity Specific heat of lead is 0.13 j/g k. For example, the specific heat capacity of water is 4,180 j/kg°c, but the. The specific heat capacity of lead. 16 rows specific heats and molar heat capacities for various substances at 20 c substance c in j/gm k c in cal/gm k or btu/lb f molar c j/mol k. The specific heat is the amount of heat energy per unit mass required to raise the temperature by. 71 rows specific heat capacity of metals table chart. For example, the heat required to raise the temperature of 1 kg of water by 1 k is 4184 joules, so the specific. How many joules of heat are required to heat 69 g of lead from 25.5 degree celsius to 85.6 degree celsius? The si unit of specific heat capacity is joule per kelvin per kilogram, j⋅kg −1 ⋅k −1. Different substances have different specific heat capacities. Specific heat of lead is 0.13 j/g k. 55 rows the table of specific heat capacities gives the volumetric heat capacity as well as the specific heat capacity of some substances and. Specific heat, or specific heat capacity, is a property related to internal energy that is very important in.
From www.sciencefacts.net
Specific Heat and Heat Capacity Definition, Formula, Values, and Problems Joules Lead Heat Capacity Specific heat of lead is 0.13 j/g k. For example, the specific heat capacity of water is 4,180 j/kg°c, but the. The si unit of specific heat capacity is joule per kelvin per kilogram, j⋅kg −1 ⋅k −1. The specific heat capacity of lead. How many joules of heat are required to heat 69 g of lead from 25.5 degree. Joules Lead Heat Capacity.
From www.careerpower.in
What is Heat Capacity Definition, Equations, Examples and Types Joules Lead Heat Capacity For example, the specific heat capacity of water is 4,180 j/kg°c, but the. 16 rows specific heats and molar heat capacities for various substances at 20 c substance c in j/gm k c in cal/gm k or btu/lb f molar c j/mol k. For example, the heat required to raise the temperature of 1 kg of water by 1 k. Joules Lead Heat Capacity.
From www.numerade.com
SOLVEDCalculate the heat capacity, in joules and in calories per Joules Lead Heat Capacity 71 rows specific heat capacity of metals table chart. The si unit of specific heat capacity is joule per kelvin per kilogram, j⋅kg −1 ⋅k −1. Different substances have different specific heat capacities. For example, the specific heat capacity of water is 4,180 j/kg°c, but the. For example, the heat required to raise the temperature of 1 kg of water. Joules Lead Heat Capacity.
From www.slideserve.com
PPT Chapter10 Temperature and Heat PowerPoint Presentation, free Joules Lead Heat Capacity 16 rows specific heats and molar heat capacities for various substances at 20 c substance c in j/gm k c in cal/gm k or btu/lb f molar c j/mol k. The si unit of specific heat capacity is joule per kelvin per kilogram, j⋅kg −1 ⋅k −1. For example, the specific heat capacity of water is 4,180 j/kg°c, but the.. Joules Lead Heat Capacity.
From scienceinfo.com
Heat Capacity and Specific Heat Joules Lead Heat Capacity How many joules of heat are required to heat 69 g of lead from 25.5 degree celsius to 85.6 degree celsius? The si unit of specific heat capacity is joule per kelvin per kilogram, j⋅kg −1 ⋅k −1. Different substances have different specific heat capacities. For example, the heat required to raise the temperature of 1 kg of water by. Joules Lead Heat Capacity.
From www.coursehero.com
[Solved] Calculate the amount of heat energy needed in joules, to Joules Lead Heat Capacity The specific heat is the amount of heat energy per unit mass required to raise the temperature by. 71 rows specific heat capacity of metals table chart. Specific heat, or specific heat capacity, is a property related to internal energy that is very important in. Specific heat of lead is 0.13 j/g k. The si unit of specific heat capacity. Joules Lead Heat Capacity.
From www.wikihow.com
How to Calculate Heat Capacity 8 Steps (with Pictures) wikiHow Joules Lead Heat Capacity Different substances have different specific heat capacities. The specific heat is the amount of heat energy per unit mass required to raise the temperature by. Specific heat of lead is 0.13 j/g k. The specific heat capacity of lead. How many joules of heat are required to heat 69 g of lead from 25.5 degree celsius to 85.6 degree celsius?. Joules Lead Heat Capacity.
From physicsomets.netlify.app
Physics Formula For Specific Heat Capacity Joules Lead Heat Capacity 55 rows the table of specific heat capacities gives the volumetric heat capacity as well as the specific heat capacity of some substances and. The si unit of specific heat capacity is joule per kelvin per kilogram, j⋅kg −1 ⋅k −1. Specific heat of lead is 0.13 j/g k. For example, the specific heat capacity of water is 4,180 j/kg°c,. Joules Lead Heat Capacity.
From www.bartleby.com
Answered HONORS ONLY 12. How many joules are… bartleby Joules Lead Heat Capacity For example, the specific heat capacity of water is 4,180 j/kg°c, but the. 71 rows specific heat capacity of metals table chart. How many joules of heat are required to heat 69 g of lead from 25.5 degree celsius to 85.6 degree celsius? 16 rows specific heats and molar heat capacities for various substances at 20 c substance c in. Joules Lead Heat Capacity.
From www.gauthmath.com
Solved Every material has a property known as specific heat capacity Joules Lead Heat Capacity The specific heat is the amount of heat energy per unit mass required to raise the temperature by. Specific heat, or specific heat capacity, is a property related to internal energy that is very important in. 16 rows specific heats and molar heat capacities for various substances at 20 c substance c in j/gm k c in cal/gm k or. Joules Lead Heat Capacity.
From www.hanlin.com
Edexcel IGCSE Physics 复习笔记 5.2.4 Specific Heat Capacity翰林国际教育 Joules Lead Heat Capacity The specific heat capacity of lead. The specific heat is the amount of heat energy per unit mass required to raise the temperature by. For example, the specific heat capacity of water is 4,180 j/kg°c, but the. 16 rows specific heats and molar heat capacities for various substances at 20 c substance c in j/gm k c in cal/gm k. Joules Lead Heat Capacity.
From www.youtube.com
The heat capacity of a substance is the amount of energy, in joules (J Joules Lead Heat Capacity The specific heat is the amount of heat energy per unit mass required to raise the temperature by. For example, the specific heat capacity of water is 4,180 j/kg°c, but the. Different substances have different specific heat capacities. The specific heat capacity of lead. 71 rows specific heat capacity of metals table chart. The si unit of specific heat capacity. Joules Lead Heat Capacity.
From www.youtube.com
Joules Calorimeter ExperimentFind the Specific heat capacity of water Joules Lead Heat Capacity 71 rows specific heat capacity of metals table chart. Specific heat of lead is 0.13 j/g k. For example, the specific heat capacity of water is 4,180 j/kg°c, but the. The specific heat is the amount of heat energy per unit mass required to raise the temperature by. 16 rows specific heats and molar heat capacities for various substances at. Joules Lead Heat Capacity.
From www.slideserve.com
PPT Specific Heat Capacity PowerPoint Presentation, free download Joules Lead Heat Capacity 16 rows specific heats and molar heat capacities for various substances at 20 c substance c in j/gm k c in cal/gm k or btu/lb f molar c j/mol k. Different substances have different specific heat capacities. The specific heat is the amount of heat energy per unit mass required to raise the temperature by. The specific heat capacity of. Joules Lead Heat Capacity.
From www.youtube.com
Specific Heat Capacity Solving for Joules YouTube Joules Lead Heat Capacity The specific heat capacity of lead. The specific heat is the amount of heat energy per unit mass required to raise the temperature by. For example, the specific heat capacity of water is 4,180 j/kg°c, but the. Specific heat of lead is 0.13 j/g k. 71 rows specific heat capacity of metals table chart. 16 rows specific heats and molar. Joules Lead Heat Capacity.
From www.numerade.com
SOLVED A 9.84 nugget of unknown metal is heated from 73 °C to 192 °C Joules Lead Heat Capacity Specific heat, or specific heat capacity, is a property related to internal energy that is very important in. Specific heat of lead is 0.13 j/g k. The si unit of specific heat capacity is joule per kelvin per kilogram, j⋅kg −1 ⋅k −1. Different substances have different specific heat capacities. For example, the specific heat capacity of water is 4,180. Joules Lead Heat Capacity.
From www.numerade.com
SOLVED Table 9.2 Specific Heat Capacities of Some Common Substances Joules Lead Heat Capacity The si unit of specific heat capacity is joule per kelvin per kilogram, j⋅kg −1 ⋅k −1. 55 rows the table of specific heat capacities gives the volumetric heat capacity as well as the specific heat capacity of some substances and. 16 rows specific heats and molar heat capacities for various substances at 20 c substance c in j/gm k. Joules Lead Heat Capacity.
From oneclass.com
OneClass The specific heat capacity of solid copper metal is 0.385 J/g Joules Lead Heat Capacity The specific heat is the amount of heat energy per unit mass required to raise the temperature by. Different substances have different specific heat capacities. 16 rows specific heats and molar heat capacities for various substances at 20 c substance c in j/gm k c in cal/gm k or btu/lb f molar c j/mol k. How many joules of heat. Joules Lead Heat Capacity.
From www.chegg.com
Solved explain why heat capacity for lead and other metals Joules Lead Heat Capacity For example, the specific heat capacity of water is 4,180 j/kg°c, but the. 71 rows specific heat capacity of metals table chart. Different substances have different specific heat capacities. The specific heat is the amount of heat energy per unit mass required to raise the temperature by. Specific heat of lead is 0.13 j/g k. How many joules of heat. Joules Lead Heat Capacity.
From www.chegg.com
Solved A bomb calorimeter has a heat capacity of 2.47 kJ/K. Joules Lead Heat Capacity Specific heat of lead is 0.13 j/g k. 16 rows specific heats and molar heat capacities for various substances at 20 c substance c in j/gm k c in cal/gm k or btu/lb f molar c j/mol k. Specific heat, or specific heat capacity, is a property related to internal energy that is very important in. For example, the heat. Joules Lead Heat Capacity.
From www.numerade.com
SOLVEDCalculate the heat capacity, in joules and in calories per Joules Lead Heat Capacity For example, the specific heat capacity of water is 4,180 j/kg°c, but the. How many joules of heat are required to heat 69 g of lead from 25.5 degree celsius to 85.6 degree celsius? Specific heat of lead is 0.13 j/g k. Specific heat, or specific heat capacity, is a property related to internal energy that is very important in.. Joules Lead Heat Capacity.
From www.chegg.com
Solved The table provides molar heat capacities (Cm) of Joules Lead Heat Capacity The specific heat is the amount of heat energy per unit mass required to raise the temperature by. 55 rows the table of specific heat capacities gives the volumetric heat capacity as well as the specific heat capacity of some substances and. Different substances have different specific heat capacities. 71 rows specific heat capacity of metals table chart. 16 rows. Joules Lead Heat Capacity.
From ar.inspiredpencil.com
Specific Heat Chart Aluminum Joules Lead Heat Capacity 55 rows the table of specific heat capacities gives the volumetric heat capacity as well as the specific heat capacity of some substances and. Specific heat, or specific heat capacity, is a property related to internal energy that is very important in. Different substances have different specific heat capacities. For example, the specific heat capacity of water is 4,180 j/kg°c,. Joules Lead Heat Capacity.
From studylib.net
Heat Capacity of Metals PreLab Joules Lead Heat Capacity Specific heat of lead is 0.13 j/g k. For example, the specific heat capacity of water is 4,180 j/kg°c, but the. How many joules of heat are required to heat 69 g of lead from 25.5 degree celsius to 85.6 degree celsius? 71 rows specific heat capacity of metals table chart. Different substances have different specific heat capacities. 55 rows. Joules Lead Heat Capacity.
From www.saralmind.com
Heat Notes, Videos, QA and Tests Class 10>Science>Heat SaralMind Joules Lead Heat Capacity 71 rows specific heat capacity of metals table chart. For example, the heat required to raise the temperature of 1 kg of water by 1 k is 4184 joules, so the specific. How many joules of heat are required to heat 69 g of lead from 25.5 degree celsius to 85.6 degree celsius? For example, the specific heat capacity of. Joules Lead Heat Capacity.
From www.chegg.com
Solved Table 1 Specific Heat Capacity of Common Substances Joules Lead Heat Capacity The specific heat is the amount of heat energy per unit mass required to raise the temperature by. 16 rows specific heats and molar heat capacities for various substances at 20 c substance c in j/gm k c in cal/gm k or btu/lb f molar c j/mol k. The si unit of specific heat capacity is joule per kelvin per. Joules Lead Heat Capacity.
From haipernews.com
How To Find Specific Heat Capacity Without Joules Haiper Joules Lead Heat Capacity How many joules of heat are required to heat 69 g of lead from 25.5 degree celsius to 85.6 degree celsius? Different substances have different specific heat capacities. 55 rows the table of specific heat capacities gives the volumetric heat capacity as well as the specific heat capacity of some substances and. Specific heat of lead is 0.13 j/g k.. Joules Lead Heat Capacity.
From www.slideserve.com
PPT HEAT EQUATION (in Table T) PowerPoint Presentation, free download Joules Lead Heat Capacity Specific heat of lead is 0.13 j/g k. For example, the heat required to raise the temperature of 1 kg of water by 1 k is 4184 joules, so the specific. Different substances have different specific heat capacities. 55 rows the table of specific heat capacities gives the volumetric heat capacity as well as the specific heat capacity of some. Joules Lead Heat Capacity.
From www.tec-science.com
Specific heat capacity of selected substances tecscience Joules Lead Heat Capacity The specific heat is the amount of heat energy per unit mass required to raise the temperature by. How many joules of heat are required to heat 69 g of lead from 25.5 degree celsius to 85.6 degree celsius? For example, the heat required to raise the temperature of 1 kg of water by 1 k is 4184 joules, so. Joules Lead Heat Capacity.
From haipernews.com
How To Find Specific Heat Capacity Without Joules Haiper Joules Lead Heat Capacity The specific heat is the amount of heat energy per unit mass required to raise the temperature by. 55 rows the table of specific heat capacities gives the volumetric heat capacity as well as the specific heat capacity of some substances and. For example, the heat required to raise the temperature of 1 kg of water by 1 k is. Joules Lead Heat Capacity.
From slideplayer.com
Chapter 16 Reaction Energy ppt download Joules Lead Heat Capacity Specific heat, or specific heat capacity, is a property related to internal energy that is very important in. 16 rows specific heats and molar heat capacities for various substances at 20 c substance c in j/gm k c in cal/gm k or btu/lb f molar c j/mol k. For example, the specific heat capacity of water is 4,180 j/kg°c, but. Joules Lead Heat Capacity.
From www.slideserve.com
PPT Specific Heat Capacity PowerPoint Presentation, free download Joules Lead Heat Capacity The si unit of specific heat capacity is joule per kelvin per kilogram, j⋅kg −1 ⋅k −1. Different substances have different specific heat capacities. 55 rows the table of specific heat capacities gives the volumetric heat capacity as well as the specific heat capacity of some substances and. For example, the heat required to raise the temperature of 1 kg. Joules Lead Heat Capacity.
From www.slideshare.net
Quantity of heat Joules Lead Heat Capacity The specific heat is the amount of heat energy per unit mass required to raise the temperature by. Different substances have different specific heat capacities. Specific heat of lead is 0.13 j/g k. The specific heat capacity of lead. How many joules of heat are required to heat 69 g of lead from 25.5 degree celsius to 85.6 degree celsius?. Joules Lead Heat Capacity.
From physicscalculations.com
How to Calculate Specific Heat Capacity Joules Lead Heat Capacity The specific heat is the amount of heat energy per unit mass required to raise the temperature by. Different substances have different specific heat capacities. 55 rows the table of specific heat capacities gives the volumetric heat capacity as well as the specific heat capacity of some substances and. Specific heat, or specific heat capacity, is a property related to. Joules Lead Heat Capacity.
From www.chegg.com
Solved The table provides molar heat capacities (Cm) of Joules Lead Heat Capacity For example, the specific heat capacity of water is 4,180 j/kg°c, but the. The specific heat capacity of lead. Specific heat of lead is 0.13 j/g k. Different substances have different specific heat capacities. Specific heat, or specific heat capacity, is a property related to internal energy that is very important in. The specific heat is the amount of heat. Joules Lead Heat Capacity.