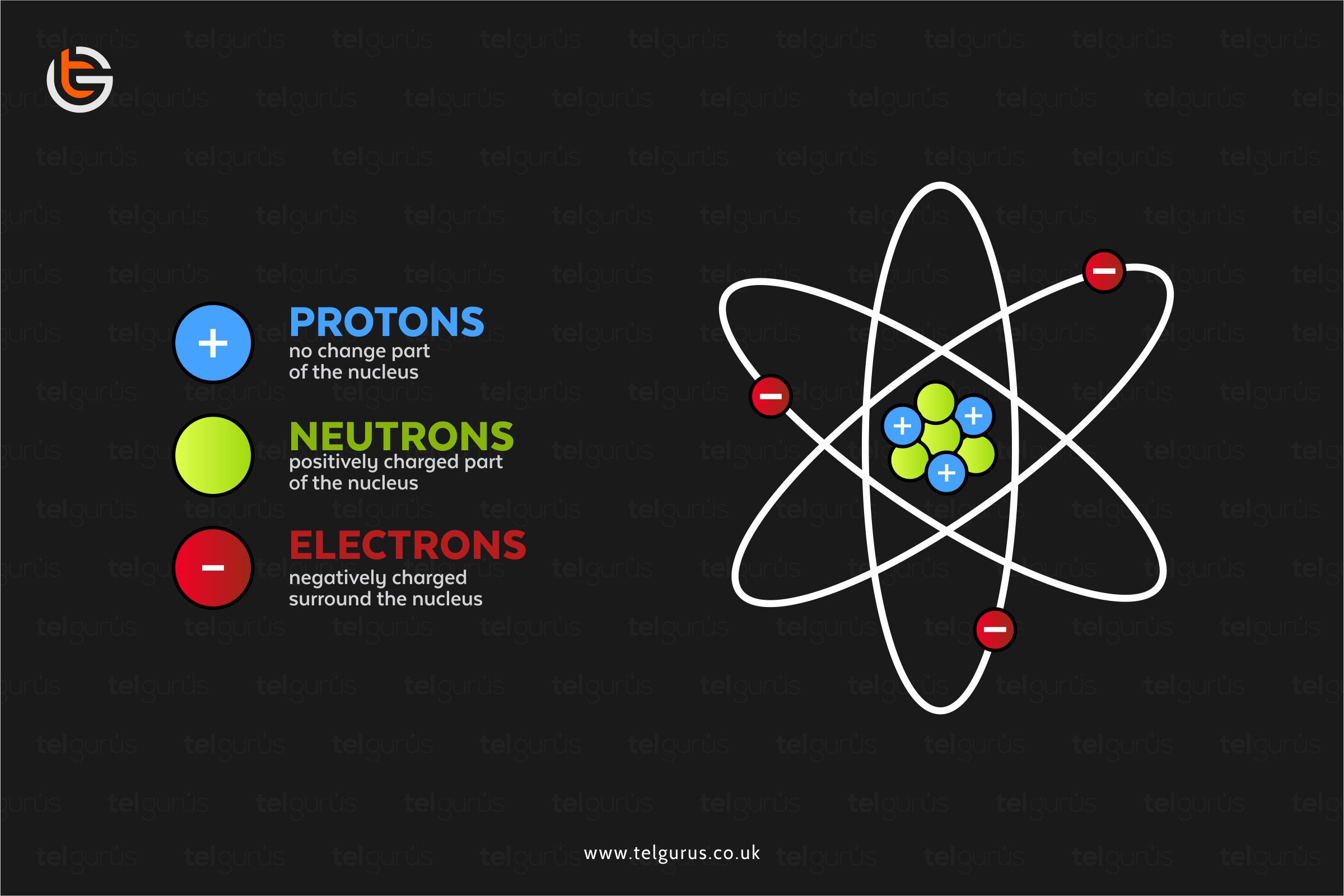
Why Do Atoms Have A Neutral Charge . Why is an atom charge neutral? The total number of protons and the total number of electrons in the nucleus of an atom balance out the total positive and. Unlike protons and electrons, which are electrically. Atoms of all elements—except for most atoms of hydrogen—have neutrons in their nucleus. Heavier atoms tend to have more neutrons than protons, but the number of electrons in an atom is always equal to the number of protons. Neutral atoms have a charge balance, which means that they have an equal number of protons and electrons. If an atom gains one or more electrons, it becomes a negatively charged ion If an atom loses one or more electrons, it becomes a positively charged ion; Most of an atom’s mass is in its. Atoms are made up of positively charged particles called protons and negatively charged particles called electrons as well as. By removing one or more electrons, neutral atoms can be converted into. The positive charges equal the negative charges, so the atom has no overall charge;
from telgurus.co.uk
If an atom gains one or more electrons, it becomes a negatively charged ion The positive charges equal the negative charges, so the atom has no overall charge; Most of an atom’s mass is in its. Atoms are made up of positively charged particles called protons and negatively charged particles called electrons as well as. Unlike protons and electrons, which are electrically. The total number of protons and the total number of electrons in the nucleus of an atom balance out the total positive and. Heavier atoms tend to have more neutrons than protons, but the number of electrons in an atom is always equal to the number of protons. Neutral atoms have a charge balance, which means that they have an equal number of protons and electrons. Atoms of all elements—except for most atoms of hydrogen—have neutrons in their nucleus. If an atom loses one or more electrons, it becomes a positively charged ion;
What is the overall charge of the nucleus of an atom and why?
Why Do Atoms Have A Neutral Charge The total number of protons and the total number of electrons in the nucleus of an atom balance out the total positive and. If an atom loses one or more electrons, it becomes a positively charged ion; The total number of protons and the total number of electrons in the nucleus of an atom balance out the total positive and. Atoms are made up of positively charged particles called protons and negatively charged particles called electrons as well as. By removing one or more electrons, neutral atoms can be converted into. Atoms of all elements—except for most atoms of hydrogen—have neutrons in their nucleus. Unlike protons and electrons, which are electrically. If an atom gains one or more electrons, it becomes a negatively charged ion The positive charges equal the negative charges, so the atom has no overall charge; Most of an atom’s mass is in its. Heavier atoms tend to have more neutrons than protons, but the number of electrons in an atom is always equal to the number of protons. Why is an atom charge neutral? Neutral atoms have a charge balance, which means that they have an equal number of protons and electrons.
From www.slideserve.com
PPT Chapter 21 Electric Charge and Electric Fields PowerPoint Why Do Atoms Have A Neutral Charge By removing one or more electrons, neutral atoms can be converted into. Most of an atom’s mass is in its. Atoms are made up of positively charged particles called protons and negatively charged particles called electrons as well as. If an atom loses one or more electrons, it becomes a positively charged ion; Why is an atom charge neutral? Atoms. Why Do Atoms Have A Neutral Charge.
From gageferscase.blogspot.com
9 Protons 10 Neutrons 10 Electrons Total Charge Why Do Atoms Have A Neutral Charge Neutral atoms have a charge balance, which means that they have an equal number of protons and electrons. The positive charges equal the negative charges, so the atom has no overall charge; If an atom gains one or more electrons, it becomes a negatively charged ion If an atom loses one or more electrons, it becomes a positively charged ion;. Why Do Atoms Have A Neutral Charge.
From www.nagwa.com
Question Video Comparing a Positive Ion to a Neutral Atom Nagwa Why Do Atoms Have A Neutral Charge Why is an atom charge neutral? Atoms are made up of positively charged particles called protons and negatively charged particles called electrons as well as. Heavier atoms tend to have more neutrons than protons, but the number of electrons in an atom is always equal to the number of protons. The positive charges equal the negative charges, so the atom. Why Do Atoms Have A Neutral Charge.
From peyton-has-tapia.blogspot.com
An Atom With a Net Positive Charge Must Have More PeytonhasTapia Why Do Atoms Have A Neutral Charge If an atom gains one or more electrons, it becomes a negatively charged ion The positive charges equal the negative charges, so the atom has no overall charge; The total number of protons and the total number of electrons in the nucleus of an atom balance out the total positive and. Most of an atom’s mass is in its. By. Why Do Atoms Have A Neutral Charge.
From www.slideserve.com
PPT History of Atomic Theory PowerPoint Presentation, free download Why Do Atoms Have A Neutral Charge Heavier atoms tend to have more neutrons than protons, but the number of electrons in an atom is always equal to the number of protons. The total number of protons and the total number of electrons in the nucleus of an atom balance out the total positive and. Most of an atom’s mass is in its. Atoms are made up. Why Do Atoms Have A Neutral Charge.
From www.youtube.com
Why is an atom electrically neutral YouTube Why Do Atoms Have A Neutral Charge If an atom loses one or more electrons, it becomes a positively charged ion; Why is an atom charge neutral? Heavier atoms tend to have more neutrons than protons, but the number of electrons in an atom is always equal to the number of protons. Neutral atoms have a charge balance, which means that they have an equal number of. Why Do Atoms Have A Neutral Charge.
From www.nagwa.com
Question Video Explaining Why Atoms Do Not Have a Charge Nagwa Why Do Atoms Have A Neutral Charge Heavier atoms tend to have more neutrons than protons, but the number of electrons in an atom is always equal to the number of protons. Atoms of all elements—except for most atoms of hydrogen—have neutrons in their nucleus. If an atom loses one or more electrons, it becomes a positively charged ion; Neutral atoms have a charge balance, which means. Why Do Atoms Have A Neutral Charge.
From learn.sparkfun.com
What is Electricity? SparkFun Learn Why Do Atoms Have A Neutral Charge Unlike protons and electrons, which are electrically. Why is an atom charge neutral? Heavier atoms tend to have more neutrons than protons, but the number of electrons in an atom is always equal to the number of protons. The total number of protons and the total number of electrons in the nucleus of an atom balance out the total positive. Why Do Atoms Have A Neutral Charge.
From www.chemistrystudent.com
Atomic Structure (ALevel) ChemistryStudent Why Do Atoms Have A Neutral Charge Atoms are made up of positively charged particles called protons and negatively charged particles called electrons as well as. Neutral atoms have a charge balance, which means that they have an equal number of protons and electrons. Atoms of all elements—except for most atoms of hydrogen—have neutrons in their nucleus. The positive charges equal the negative charges, so the atom. Why Do Atoms Have A Neutral Charge.
From www.slideserve.com
PPT Atoms have NO overall charge PowerPoint Presentation, free Why Do Atoms Have A Neutral Charge Atoms of all elements—except for most atoms of hydrogen—have neutrons in their nucleus. Atoms are made up of positively charged particles called protons and negatively charged particles called electrons as well as. Heavier atoms tend to have more neutrons than protons, but the number of electrons in an atom is always equal to the number of protons. If an atom. Why Do Atoms Have A Neutral Charge.
From chemistrylearninghub.com
atom is electrically neutral...WHY? CHEMISTRY LEARNING HUB Why Do Atoms Have A Neutral Charge Heavier atoms tend to have more neutrons than protons, but the number of electrons in an atom is always equal to the number of protons. If an atom loses one or more electrons, it becomes a positively charged ion; By removing one or more electrons, neutral atoms can be converted into. If an atom gains one or more electrons, it. Why Do Atoms Have A Neutral Charge.
From www.slideserve.com
PPT Lecture 1 Charge and Coulomb’s Law PowerPoint Presentation, free Why Do Atoms Have A Neutral Charge The total number of protons and the total number of electrons in the nucleus of an atom balance out the total positive and. Most of an atom’s mass is in its. Unlike protons and electrons, which are electrically. If an atom gains one or more electrons, it becomes a negatively charged ion The positive charges equal the negative charges, so. Why Do Atoms Have A Neutral Charge.
From www.youtube.com
Why Are Atoms Neutral GCSE Chemistry YouTube Why Do Atoms Have A Neutral Charge By removing one or more electrons, neutral atoms can be converted into. If an atom loses one or more electrons, it becomes a positively charged ion; Atoms are made up of positively charged particles called protons and negatively charged particles called electrons as well as. The positive charges equal the negative charges, so the atom has no overall charge; The. Why Do Atoms Have A Neutral Charge.
From electricallive.com
Atomic Structure And Electric Charge Electrical engineering interview Why Do Atoms Have A Neutral Charge The total number of protons and the total number of electrons in the nucleus of an atom balance out the total positive and. Unlike protons and electrons, which are electrically. If an atom gains one or more electrons, it becomes a negatively charged ion By removing one or more electrons, neutral atoms can be converted into. If an atom loses. Why Do Atoms Have A Neutral Charge.
From classfullplymouth.z21.web.core.windows.net
Parts Of The Atom Why Do Atoms Have A Neutral Charge Atoms of all elements—except for most atoms of hydrogen—have neutrons in their nucleus. By removing one or more electrons, neutral atoms can be converted into. Heavier atoms tend to have more neutrons than protons, but the number of electrons in an atom is always equal to the number of protons. Most of an atom’s mass is in its. The positive. Why Do Atoms Have A Neutral Charge.
From www.slideserve.com
PPT Mon 9/24 and Tues 9/25 PowerPoint Presentation ID1563791 Why Do Atoms Have A Neutral Charge The positive charges equal the negative charges, so the atom has no overall charge; The total number of protons and the total number of electrons in the nucleus of an atom balance out the total positive and. Neutral atoms have a charge balance, which means that they have an equal number of protons and electrons. Atoms are made up of. Why Do Atoms Have A Neutral Charge.
From www.slideserve.com
PPT Types of Chemical Bonds PowerPoint Presentation, free download Why Do Atoms Have A Neutral Charge The total number of protons and the total number of electrons in the nucleus of an atom balance out the total positive and. Unlike protons and electrons, which are electrically. By removing one or more electrons, neutral atoms can be converted into. Most of an atom’s mass is in its. The positive charges equal the negative charges, so the atom. Why Do Atoms Have A Neutral Charge.
From www.animalia-life.club
Electrons In An Atom Why Do Atoms Have A Neutral Charge Neutral atoms have a charge balance, which means that they have an equal number of protons and electrons. Atoms of all elements—except for most atoms of hydrogen—have neutrons in their nucleus. Unlike protons and electrons, which are electrically. Atoms are made up of positively charged particles called protons and negatively charged particles called electrons as well as. The positive charges. Why Do Atoms Have A Neutral Charge.
From warstek.com
BagianBagian Atom Warung Sains Teknologi Why Do Atoms Have A Neutral Charge The positive charges equal the negative charges, so the atom has no overall charge; If an atom loses one or more electrons, it becomes a positively charged ion; If an atom gains one or more electrons, it becomes a negatively charged ion Atoms of all elements—except for most atoms of hydrogen—have neutrons in their nucleus. Heavier atoms tend to have. Why Do Atoms Have A Neutral Charge.
From www.slideserve.com
PPT Ion Formation PowerPoint Presentation ID2508414 Why Do Atoms Have A Neutral Charge If an atom loses one or more electrons, it becomes a positively charged ion; Atoms are made up of positively charged particles called protons and negatively charged particles called electrons as well as. Unlike protons and electrons, which are electrically. Neutral atoms have a charge balance, which means that they have an equal number of protons and electrons. Atoms of. Why Do Atoms Have A Neutral Charge.
From thebiologyprimer.com
Atoms & Molecules echapter — The Biology Primer Why Do Atoms Have A Neutral Charge The total number of protons and the total number of electrons in the nucleus of an atom balance out the total positive and. Most of an atom’s mass is in its. Unlike protons and electrons, which are electrically. Atoms of all elements—except for most atoms of hydrogen—have neutrons in their nucleus. Why is an atom charge neutral? If an atom. Why Do Atoms Have A Neutral Charge.
From learn.sparkfun.com
What is Electricity? SparkFun Learn Why Do Atoms Have A Neutral Charge The total number of protons and the total number of electrons in the nucleus of an atom balance out the total positive and. The positive charges equal the negative charges, so the atom has no overall charge; Unlike protons and electrons, which are electrically. Why is an atom charge neutral? Most of an atom’s mass is in its. Atoms of. Why Do Atoms Have A Neutral Charge.
From www.youtube.com
Why atom is electrically neutral 🤷♂ YouTube Why Do Atoms Have A Neutral Charge Heavier atoms tend to have more neutrons than protons, but the number of electrons in an atom is always equal to the number of protons. If an atom gains one or more electrons, it becomes a negatively charged ion Unlike protons and electrons, which are electrically. Atoms of all elements—except for most atoms of hydrogen—have neutrons in their nucleus. Most. Why Do Atoms Have A Neutral Charge.
From www.expii.com
Atoms — Definition & Overview Expii Why Do Atoms Have A Neutral Charge If an atom gains one or more electrons, it becomes a negatively charged ion Atoms of all elements—except for most atoms of hydrogen—have neutrons in their nucleus. Atoms are made up of positively charged particles called protons and negatively charged particles called electrons as well as. Neutral atoms have a charge balance, which means that they have an equal number. Why Do Atoms Have A Neutral Charge.
From sciencenotes.org
What Is the Difference Between an Atom and an Ion? Why Do Atoms Have A Neutral Charge Heavier atoms tend to have more neutrons than protons, but the number of electrons in an atom is always equal to the number of protons. By removing one or more electrons, neutral atoms can be converted into. Atoms of all elements—except for most atoms of hydrogen—have neutrons in their nucleus. If an atom gains one or more electrons, it becomes. Why Do Atoms Have A Neutral Charge.
From www.organicchemistrytutor.com
Formal Charges — Organic Chemistry Tutor Why Do Atoms Have A Neutral Charge Atoms are made up of positively charged particles called protons and negatively charged particles called electrons as well as. By removing one or more electrons, neutral atoms can be converted into. The positive charges equal the negative charges, so the atom has no overall charge; If an atom loses one or more electrons, it becomes a positively charged ion; Unlike. Why Do Atoms Have A Neutral Charge.
From www.sliderbase.com
Formula of Ionic Compounds Why Do Atoms Have A Neutral Charge The positive charges equal the negative charges, so the atom has no overall charge; Heavier atoms tend to have more neutrons than protons, but the number of electrons in an atom is always equal to the number of protons. Atoms are made up of positively charged particles called protons and negatively charged particles called electrons as well as. Unlike protons. Why Do Atoms Have A Neutral Charge.
From solbergsphyscience.wikispaces.com
solbergsphyscience The Structure of the Atom Why Do Atoms Have A Neutral Charge The total number of protons and the total number of electrons in the nucleus of an atom balance out the total positive and. Unlike protons and electrons, which are electrically. By removing one or more electrons, neutral atoms can be converted into. The positive charges equal the negative charges, so the atom has no overall charge; If an atom loses. Why Do Atoms Have A Neutral Charge.
From slideplayer.com
Warmup Draw an atom. ppt download Why Do Atoms Have A Neutral Charge The total number of protons and the total number of electrons in the nucleus of an atom balance out the total positive and. Why is an atom charge neutral? Atoms of all elements—except for most atoms of hydrogen—have neutrons in their nucleus. Heavier atoms tend to have more neutrons than protons, but the number of electrons in an atom is. Why Do Atoms Have A Neutral Charge.
From www.youtube.com
Neutral Atoms, Ions, and Isotopes YouTube Why Do Atoms Have A Neutral Charge Why is an atom charge neutral? The total number of protons and the total number of electrons in the nucleus of an atom balance out the total positive and. Most of an atom’s mass is in its. The positive charges equal the negative charges, so the atom has no overall charge; Unlike protons and electrons, which are electrically. If an. Why Do Atoms Have A Neutral Charge.
From www.slideserve.com
PPT Ion Formation PowerPoint Presentation, free download ID2508414 Why Do Atoms Have A Neutral Charge The total number of protons and the total number of electrons in the nucleus of an atom balance out the total positive and. The positive charges equal the negative charges, so the atom has no overall charge; Unlike protons and electrons, which are electrically. Heavier atoms tend to have more neutrons than protons, but the number of electrons in an. Why Do Atoms Have A Neutral Charge.
From telgurus.co.uk
What is the overall charge of the nucleus of an atom and why? Why Do Atoms Have A Neutral Charge If an atom gains one or more electrons, it becomes a negatively charged ion Atoms are made up of positively charged particles called protons and negatively charged particles called electrons as well as. Most of an atom’s mass is in its. Why is an atom charge neutral? Unlike protons and electrons, which are electrically. The total number of protons and. Why Do Atoms Have A Neutral Charge.
From sciencenotes.org
Are Two Atoms of the Same Element Identical? Why Do Atoms Have A Neutral Charge Unlike protons and electrons, which are electrically. The positive charges equal the negative charges, so the atom has no overall charge; Most of an atom’s mass is in its. Neutral atoms have a charge balance, which means that they have an equal number of protons and electrons. Atoms of all elements—except for most atoms of hydrogen—have neutrons in their nucleus.. Why Do Atoms Have A Neutral Charge.
From www.scienceabc.com
Octet Rule Definition, Explanation, Exceptions And Examples Why Do Atoms Have A Neutral Charge By removing one or more electrons, neutral atoms can be converted into. The positive charges equal the negative charges, so the atom has no overall charge; Heavier atoms tend to have more neutrons than protons, but the number of electrons in an atom is always equal to the number of protons. If an atom loses one or more electrons, it. Why Do Atoms Have A Neutral Charge.
From www.slideserve.com
PPT The atom PowerPoint Presentation ID2479579 Why Do Atoms Have A Neutral Charge By removing one or more electrons, neutral atoms can be converted into. The positive charges equal the negative charges, so the atom has no overall charge; Unlike protons and electrons, which are electrically. Atoms are made up of positively charged particles called protons and negatively charged particles called electrons as well as. Heavier atoms tend to have more neutrons than. Why Do Atoms Have A Neutral Charge.