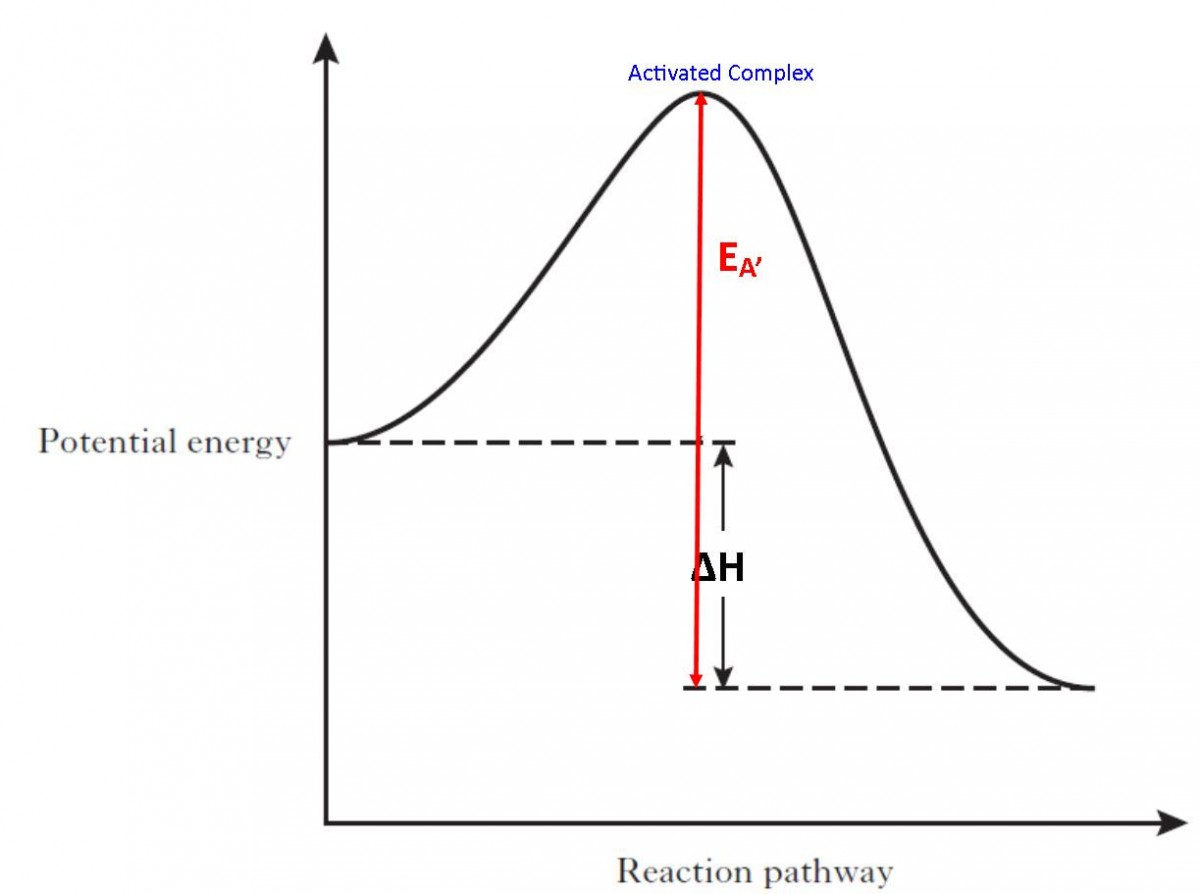
Define Threshold Energy And Activation Energy. How Are They Related . The activation energy $e_a$ of a reaction is the amount of energy required to take reactants to products across some reaction coordinate. In chemistry and physics, activation energy is the minimum amount of energy needed to start a chemical reaction. Activation energy and threshold energy are both concepts used in chemical reactions. Activation energy is the minimum energy required for a reaction to occur, while threshold energy is the total energy needed to. The difference between these two values is the activation energy,. Reactants often get activation energy from heat,. It is the minimum amount of energy which the reactant molecules must possess for the effective. The threshold energy is the energy that these molecules must have in order for a reaction to take place. Threshold energy= average of the initial kinetic energy possessed by the reactants + activation energy (ea) if the reactants at the start of the. Activation energy refers to the minimum amount of energy.
from blogs.glowscotland.org.uk
Activation energy and threshold energy are both concepts used in chemical reactions. In chemistry and physics, activation energy is the minimum amount of energy needed to start a chemical reaction. Reactants often get activation energy from heat,. The activation energy $e_a$ of a reaction is the amount of energy required to take reactants to products across some reaction coordinate. It is the minimum amount of energy which the reactant molecules must possess for the effective. The difference between these two values is the activation energy,. Activation energy is the minimum energy required for a reaction to occur, while threshold energy is the total energy needed to. Threshold energy= average of the initial kinetic energy possessed by the reactants + activation energy (ea) if the reactants at the start of the. Activation energy refers to the minimum amount of energy. The threshold energy is the energy that these molecules must have in order for a reaction to take place.
Activation Energy Higher Chemistry Unit 1
Define Threshold Energy And Activation Energy. How Are They Related Reactants often get activation energy from heat,. The activation energy $e_a$ of a reaction is the amount of energy required to take reactants to products across some reaction coordinate. The difference between these two values is the activation energy,. It is the minimum amount of energy which the reactant molecules must possess for the effective. Reactants often get activation energy from heat,. Threshold energy= average of the initial kinetic energy possessed by the reactants + activation energy (ea) if the reactants at the start of the. Activation energy and threshold energy are both concepts used in chemical reactions. The threshold energy is the energy that these molecules must have in order for a reaction to take place. Activation energy is the minimum energy required for a reaction to occur, while threshold energy is the total energy needed to. Activation energy refers to the minimum amount of energy. In chemistry and physics, activation energy is the minimum amount of energy needed to start a chemical reaction.
From www.doubtnut.com
[Punjabi] Define threshold energy and activation energy. How are they Define Threshold Energy And Activation Energy. How Are They Related The threshold energy is the energy that these molecules must have in order for a reaction to take place. Activation energy and threshold energy are both concepts used in chemical reactions. Reactants often get activation energy from heat,. It is the minimum amount of energy which the reactant molecules must possess for the effective. The difference between these two values. Define Threshold Energy And Activation Energy. How Are They Related.
From www.britannica.com
Activation energy Definition & Facts Britannica Define Threshold Energy And Activation Energy. How Are They Related The threshold energy is the energy that these molecules must have in order for a reaction to take place. The activation energy $e_a$ of a reaction is the amount of energy required to take reactants to products across some reaction coordinate. Activation energy refers to the minimum amount of energy. It is the minimum amount of energy which the reactant. Define Threshold Energy And Activation Energy. How Are They Related.
From www.alamy.com
Graph of Progress of Reaction and Threshold energy Stock Vector Image Define Threshold Energy And Activation Energy. How Are They Related In chemistry and physics, activation energy is the minimum amount of energy needed to start a chemical reaction. Threshold energy= average of the initial kinetic energy possessed by the reactants + activation energy (ea) if the reactants at the start of the. Activation energy and threshold energy are both concepts used in chemical reactions. The difference between these two values. Define Threshold Energy And Activation Energy. How Are They Related.
From nesslabs.com
Activation energy the chemistry of getting started Ness Labs Define Threshold Energy And Activation Energy. How Are They Related The difference between these two values is the activation energy,. In chemistry and physics, activation energy is the minimum amount of energy needed to start a chemical reaction. Activation energy refers to the minimum amount of energy. Activation energy is the minimum energy required for a reaction to occur, while threshold energy is the total energy needed to. The threshold. Define Threshold Energy And Activation Energy. How Are They Related.
From www.expii.com
Activation Energy — Definition & Importance Expii Define Threshold Energy And Activation Energy. How Are They Related The threshold energy is the energy that these molecules must have in order for a reaction to take place. Activation energy and threshold energy are both concepts used in chemical reactions. Activation energy refers to the minimum amount of energy. The activation energy $e_a$ of a reaction is the amount of energy required to take reactants to products across some. Define Threshold Energy And Activation Energy. How Are They Related.
From design1systems.com
The Role of Activation Energy in Potential Energy Diagrams Define Threshold Energy And Activation Energy. How Are They Related Threshold energy= average of the initial kinetic energy possessed by the reactants + activation energy (ea) if the reactants at the start of the. Activation energy is the minimum energy required for a reaction to occur, while threshold energy is the total energy needed to. The activation energy $e_a$ of a reaction is the amount of energy required to take. Define Threshold Energy And Activation Energy. How Are They Related.
From www.vrogue.co
Activation Energy Definition Formula Diagram Examples vrogue.co Define Threshold Energy And Activation Energy. How Are They Related Activation energy is the minimum energy required for a reaction to occur, while threshold energy is the total energy needed to. The threshold energy is the energy that these molecules must have in order for a reaction to take place. Activation energy and threshold energy are both concepts used in chemical reactions. Activation energy refers to the minimum amount of. Define Threshold Energy And Activation Energy. How Are They Related.
From byjus.com
Activation Energy Definition, Formula, SI Units, Examples, Calculation Define Threshold Energy And Activation Energy. How Are They Related The difference between these two values is the activation energy,. Reactants often get activation energy from heat,. Threshold energy= average of the initial kinetic energy possessed by the reactants + activation energy (ea) if the reactants at the start of the. Activation energy and threshold energy are both concepts used in chemical reactions. In chemistry and physics, activation energy is. Define Threshold Energy And Activation Energy. How Are They Related.
From www.chemistrylearner.com
Activation Energy Definition, Formula, and Graph Define Threshold Energy And Activation Energy. How Are They Related Activation energy is the minimum energy required for a reaction to occur, while threshold energy is the total energy needed to. In chemistry and physics, activation energy is the minimum amount of energy needed to start a chemical reaction. The difference between these two values is the activation energy,. Threshold energy= average of the initial kinetic energy possessed by the. Define Threshold Energy And Activation Energy. How Are They Related.
From bio.libretexts.org
3.9 Energy in Chemical Reactions Biology LibreTexts Define Threshold Energy And Activation Energy. How Are They Related The activation energy $e_a$ of a reaction is the amount of energy required to take reactants to products across some reaction coordinate. Activation energy refers to the minimum amount of energy. It is the minimum amount of energy which the reactant molecules must possess for the effective. The difference between these two values is the activation energy,. In chemistry and. Define Threshold Energy And Activation Energy. How Are They Related.
From sciencenotes.org
What Is Activation Energy? Definition and Examples Define Threshold Energy And Activation Energy. How Are They Related Threshold energy= average of the initial kinetic energy possessed by the reactants + activation energy (ea) if the reactants at the start of the. The activation energy $e_a$ of a reaction is the amount of energy required to take reactants to products across some reaction coordinate. Activation energy and threshold energy are both concepts used in chemical reactions. It is. Define Threshold Energy And Activation Energy. How Are They Related.
From www.youtube.com
Difference between Activation energy and Threshold energy YouTube Define Threshold Energy And Activation Energy. How Are They Related The threshold energy is the energy that these molecules must have in order for a reaction to take place. Activation energy and threshold energy are both concepts used in chemical reactions. In chemistry and physics, activation energy is the minimum amount of energy needed to start a chemical reaction. Reactants often get activation energy from heat,. Threshold energy= average of. Define Threshold Energy And Activation Energy. How Are They Related.
From www.doubtnut.com
[Odia] Define threshold energy and activation energy. How are they rel Define Threshold Energy And Activation Energy. How Are They Related The activation energy $e_a$ of a reaction is the amount of energy required to take reactants to products across some reaction coordinate. In chemistry and physics, activation energy is the minimum amount of energy needed to start a chemical reaction. The difference between these two values is the activation energy,. It is the minimum amount of energy which the reactant. Define Threshold Energy And Activation Energy. How Are They Related.
From blogs.glowscotland.org.uk
Activation Energy Higher Chemistry Unit 1 Define Threshold Energy And Activation Energy. How Are They Related Reactants often get activation energy from heat,. The threshold energy is the energy that these molecules must have in order for a reaction to take place. The difference between these two values is the activation energy,. Activation energy is the minimum energy required for a reaction to occur, while threshold energy is the total energy needed to. Threshold energy= average. Define Threshold Energy And Activation Energy. How Are They Related.
From byjus.com
What happens when the energy of a reaction if more than threshold Define Threshold Energy And Activation Energy. How Are They Related Activation energy is the minimum energy required for a reaction to occur, while threshold energy is the total energy needed to. In chemistry and physics, activation energy is the minimum amount of energy needed to start a chemical reaction. It is the minimum amount of energy which the reactant molecules must possess for the effective. The threshold energy is the. Define Threshold Energy And Activation Energy. How Are They Related.
From byjus.com
Activation Energy Definition, Formula, SI Units, Examples, Calculation Define Threshold Energy And Activation Energy. How Are They Related The difference between these two values is the activation energy,. The activation energy $e_a$ of a reaction is the amount of energy required to take reactants to products across some reaction coordinate. The threshold energy is the energy that these molecules must have in order for a reaction to take place. Threshold energy= average of the initial kinetic energy possessed. Define Threshold Energy And Activation Energy. How Are They Related.
From www.kosmotime.com
Activation Energy The Secret to Getting Started and Getting Finished Define Threshold Energy And Activation Energy. How Are They Related In chemistry and physics, activation energy is the minimum amount of energy needed to start a chemical reaction. The activation energy $e_a$ of a reaction is the amount of energy required to take reactants to products across some reaction coordinate. Activation energy is the minimum energy required for a reaction to occur, while threshold energy is the total energy needed. Define Threshold Energy And Activation Energy. How Are They Related.
From www.logiota.com
Activation Energy Chemical Physical Chemistry Chemistry Define Threshold Energy And Activation Energy. How Are They Related Activation energy is the minimum energy required for a reaction to occur, while threshold energy is the total energy needed to. Activation energy refers to the minimum amount of energy. The activation energy $e_a$ of a reaction is the amount of energy required to take reactants to products across some reaction coordinate. Threshold energy= average of the initial kinetic energy. Define Threshold Energy And Activation Energy. How Are They Related.
From byjus.com
Activation Energy Definition, Formula, SI Units, Examples, Calculation Define Threshold Energy And Activation Energy. How Are They Related The difference between these two values is the activation energy,. The threshold energy is the energy that these molecules must have in order for a reaction to take place. Activation energy and threshold energy are both concepts used in chemical reactions. Threshold energy= average of the initial kinetic energy possessed by the reactants + activation energy (ea) if the reactants. Define Threshold Energy And Activation Energy. How Are They Related.
From www.youtube.com
25) Activation Energy and Threshold Energy Class12 Chemical Define Threshold Energy And Activation Energy. How Are They Related The difference between these two values is the activation energy,. Activation energy refers to the minimum amount of energy. Activation energy and threshold energy are both concepts used in chemical reactions. Reactants often get activation energy from heat,. It is the minimum amount of energy which the reactant molecules must possess for the effective. In chemistry and physics, activation energy. Define Threshold Energy And Activation Energy. How Are They Related.
From douglasnewssantana.blogspot.com
Activation Energy Can Be Described as Define Threshold Energy And Activation Energy. How Are They Related The threshold energy is the energy that these molecules must have in order for a reaction to take place. Threshold energy= average of the initial kinetic energy possessed by the reactants + activation energy (ea) if the reactants at the start of the. The difference between these two values is the activation energy,. Activation energy refers to the minimum amount. Define Threshold Energy And Activation Energy. How Are They Related.
From studyonline.blog
What Is Activation Energy? Definition and Examples Define Threshold Energy And Activation Energy. How Are They Related Threshold energy= average of the initial kinetic energy possessed by the reactants + activation energy (ea) if the reactants at the start of the. The activation energy $e_a$ of a reaction is the amount of energy required to take reactants to products across some reaction coordinate. It is the minimum amount of energy which the reactant molecules must possess for. Define Threshold Energy And Activation Energy. How Are They Related.
From www.differencebetween.com
Difference Between Activation Energy and Threshold Energy Compare the Define Threshold Energy And Activation Energy. How Are They Related The threshold energy is the energy that these molecules must have in order for a reaction to take place. Threshold energy= average of the initial kinetic energy possessed by the reactants + activation energy (ea) if the reactants at the start of the. Activation energy is the minimum energy required for a reaction to occur, while threshold energy is the. Define Threshold Energy And Activation Energy. How Are They Related.
From www.expii.com
Activation Energy — Definition & Importance Expii Define Threshold Energy And Activation Energy. How Are They Related The activation energy $e_a$ of a reaction is the amount of energy required to take reactants to products across some reaction coordinate. Activation energy refers to the minimum amount of energy. Reactants often get activation energy from heat,. Activation energy and threshold energy are both concepts used in chemical reactions. Threshold energy= average of the initial kinetic energy possessed by. Define Threshold Energy And Activation Energy. How Are They Related.
From www.ck12.org
Activation Energy ( Video ) Chemistry CK12 Foundation Define Threshold Energy And Activation Energy. How Are They Related In chemistry and physics, activation energy is the minimum amount of energy needed to start a chemical reaction. Reactants often get activation energy from heat,. It is the minimum amount of energy which the reactant molecules must possess for the effective. Activation energy is the minimum energy required for a reaction to occur, while threshold energy is the total energy. Define Threshold Energy And Activation Energy. How Are They Related.
From fixportillobadgered.z21.web.core.windows.net
Energy Diagram Activation Energy Define Threshold Energy And Activation Energy. How Are They Related Activation energy is the minimum energy required for a reaction to occur, while threshold energy is the total energy needed to. Activation energy and threshold energy are both concepts used in chemical reactions. The activation energy $e_a$ of a reaction is the amount of energy required to take reactants to products across some reaction coordinate. Activation energy refers to the. Define Threshold Energy And Activation Energy. How Are They Related.
From www.slideserve.com
PPT Activation Energy … PowerPoint Presentation, free download ID Define Threshold Energy And Activation Energy. How Are They Related The threshold energy is the energy that these molecules must have in order for a reaction to take place. Reactants often get activation energy from heat,. The difference between these two values is the activation energy,. In chemistry and physics, activation energy is the minimum amount of energy needed to start a chemical reaction. Activation energy and threshold energy are. Define Threshold Energy And Activation Energy. How Are They Related.
From www.chemistrylearner.com
Activation Energy Definition, Formula, and Graph Define Threshold Energy And Activation Energy. How Are They Related Activation energy and threshold energy are both concepts used in chemical reactions. It is the minimum amount of energy which the reactant molecules must possess for the effective. Reactants often get activation energy from heat,. The activation energy $e_a$ of a reaction is the amount of energy required to take reactants to products across some reaction coordinate. The threshold energy. Define Threshold Energy And Activation Energy. How Are They Related.
From www.differencebetween.com
Difference Between Activation Energy and Threshold Energy Compare the Define Threshold Energy And Activation Energy. How Are They Related The threshold energy is the energy that these molecules must have in order for a reaction to take place. Threshold energy= average of the initial kinetic energy possessed by the reactants + activation energy (ea) if the reactants at the start of the. Activation energy and threshold energy are both concepts used in chemical reactions. The activation energy $e_a$ of. Define Threshold Energy And Activation Energy. How Are They Related.
From www.youtube.com
What is activation energy Threshold energy energy barrier rate of Define Threshold Energy And Activation Energy. How Are They Related The threshold energy is the energy that these molecules must have in order for a reaction to take place. The difference between these two values is the activation energy,. Reactants often get activation energy from heat,. In chemistry and physics, activation energy is the minimum amount of energy needed to start a chemical reaction. Threshold energy= average of the initial. Define Threshold Energy And Activation Energy. How Are They Related.
From www.youtube.com
R2.2.4 Activation energy YouTube Define Threshold Energy And Activation Energy. How Are They Related Reactants often get activation energy from heat,. Activation energy refers to the minimum amount of energy. Threshold energy= average of the initial kinetic energy possessed by the reactants + activation energy (ea) if the reactants at the start of the. The activation energy $e_a$ of a reaction is the amount of energy required to take reactants to products across some. Define Threshold Energy And Activation Energy. How Are They Related.
From byjus.com
26. What is difference between threshold energy and activation energy? Define Threshold Energy And Activation Energy. How Are They Related The threshold energy is the energy that these molecules must have in order for a reaction to take place. Activation energy and threshold energy are both concepts used in chemical reactions. Activation energy is the minimum energy required for a reaction to occur, while threshold energy is the total energy needed to. The activation energy $e_a$ of a reaction is. Define Threshold Energy And Activation Energy. How Are They Related.
From www.nuclear-power.com
Critical Energy Threshold Energy for Fission Define Threshold Energy And Activation Energy. How Are They Related Activation energy refers to the minimum amount of energy. Activation energy is the minimum energy required for a reaction to occur, while threshold energy is the total energy needed to. The difference between these two values is the activation energy,. Activation energy and threshold energy are both concepts used in chemical reactions. The activation energy $e_a$ of a reaction is. Define Threshold Energy And Activation Energy. How Are They Related.
From scienceinfo.com
Activation Energy Definition, Unit, Formula, Calculations Define Threshold Energy And Activation Energy. How Are They Related Activation energy and threshold energy are both concepts used in chemical reactions. The threshold energy is the energy that these molecules must have in order for a reaction to take place. Threshold energy= average of the initial kinetic energy possessed by the reactants + activation energy (ea) if the reactants at the start of the. Activation energy refers to the. Define Threshold Energy And Activation Energy. How Are They Related.
From byjus.com
What happens when the energy of a reaction if more than threshold Define Threshold Energy And Activation Energy. How Are They Related Activation energy and threshold energy are both concepts used in chemical reactions. Reactants often get activation energy from heat,. Activation energy is the minimum energy required for a reaction to occur, while threshold energy is the total energy needed to. It is the minimum amount of energy which the reactant molecules must possess for the effective. Threshold energy= average of. Define Threshold Energy And Activation Energy. How Are They Related.