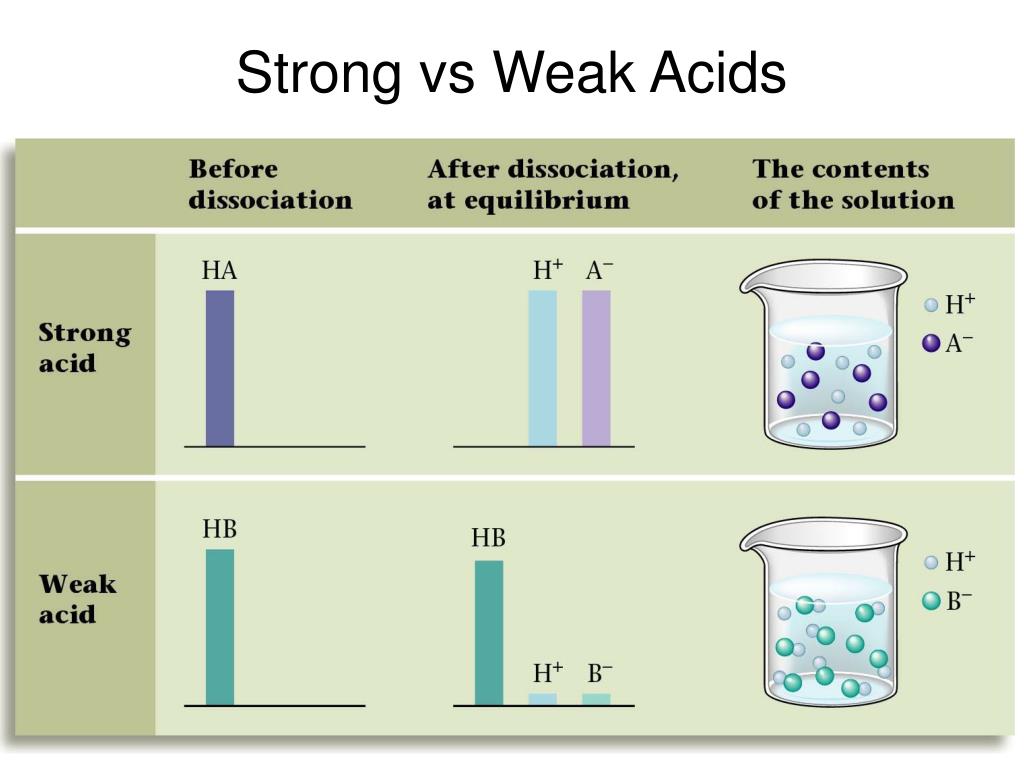
Is Hf Strong Or Weak Acid . Hc 2 h 3 o 2. It's corrosive to bones because of its fluoride. The strong acids are hydrochloric acid, nitric acid, sulfuric acid, hydrobromic acid, hydroiodic acid, perchloric acid, and chloric acid. The only weak acid formed by the reaction. Hydrofluoric acid or hf is an extremely powerful, corrosive acid. Any acid that dissociates 100% into ions is called a strong acid. However, it is a weak acid and not a strong acid because. Hydrofluoric acid is a weak acid compared to many others, but its health risks are particularly severe due to its ability to penetrate tissue. However, it’s classified as a weak acid rather than a strong acid. This makes hf the only hydrohalic acid. If it does not dissociate 100%, it is a weak acid. Hydrofluoric acid or hf is an extremely corrosive acid. Hf is corrosive because of its proton; Hf corrodes glass and hcl doesn't because silicon.
from www.slideserve.com
The only weak acid formed by the reaction. Any acid that dissociates 100% into ions is called a strong acid. The strong acids are hydrochloric acid, nitric acid, sulfuric acid, hydrobromic acid, hydroiodic acid, perchloric acid, and chloric acid. Hf corrodes glass and hcl doesn't because silicon. Hydrofluoric acid is a weak acid compared to many others, but its health risks are particularly severe due to its ability to penetrate tissue. Hf is corrosive because of its proton; However, it’s classified as a weak acid rather than a strong acid. Hydrofluoric acid or hf is an extremely powerful, corrosive acid. However, it is a weak acid and not a strong acid because. Hc 2 h 3 o 2.
PPT Ionization Constants of Acids and Bases Strengths of Acids and
Is Hf Strong Or Weak Acid Any acid that dissociates 100% into ions is called a strong acid. This makes hf the only hydrohalic acid. Hydrofluoric acid or hf is an extremely powerful, corrosive acid. Hf is corrosive because of its proton; Hf corrodes glass and hcl doesn't because silicon. Hydrofluoric acid is a weak acid compared to many others, but its health risks are particularly severe due to its ability to penetrate tissue. The only weak acid formed by the reaction. Hydrofluoric acid or hf is an extremely corrosive acid. Hc 2 h 3 o 2. It's corrosive to bones because of its fluoride. The strong acids are hydrochloric acid, nitric acid, sulfuric acid, hydrobromic acid, hydroiodic acid, perchloric acid, and chloric acid. However, it is a weak acid and not a strong acid because. If it does not dissociate 100%, it is a weak acid. However, it’s classified as a weak acid rather than a strong acid. Any acid that dissociates 100% into ions is called a strong acid.
From www.slideserve.com
PPT Acids & Bases PowerPoint Presentation ID6155898 Is Hf Strong Or Weak Acid Hf is corrosive because of its proton; Hydrofluoric acid or hf is an extremely corrosive acid. However, it is a weak acid and not a strong acid because. Hydrofluoric acid is a weak acid compared to many others, but its health risks are particularly severe due to its ability to penetrate tissue. It's corrosive to bones because of its fluoride.. Is Hf Strong Or Weak Acid.
From www.youtube.com
Is HI (Hydroiodic acid) a Strong or Weak Acid YouTube Is Hf Strong Or Weak Acid The only weak acid formed by the reaction. It's corrosive to bones because of its fluoride. Hc 2 h 3 o 2. However, it’s classified as a weak acid rather than a strong acid. Hydrofluoric acid is a weak acid compared to many others, but its health risks are particularly severe due to its ability to penetrate tissue. The strong. Is Hf Strong Or Weak Acid.
From www.slideserve.com
PPT Ch.9 J.C. Rowe PowerPoint Presentation, free download ID3493976 Is Hf Strong Or Weak Acid If it does not dissociate 100%, it is a weak acid. The only weak acid formed by the reaction. The strong acids are hydrochloric acid, nitric acid, sulfuric acid, hydrobromic acid, hydroiodic acid, perchloric acid, and chloric acid. Hydrofluoric acid or hf is an extremely corrosive acid. Hydrofluoric acid or hf is an extremely powerful, corrosive acid. Any acid that. Is Hf Strong Or Weak Acid.
From www.sliderbase.com
Acids, pH and Equilibrium Presentation Chemistry Is Hf Strong Or Weak Acid Hydrofluoric acid or hf is an extremely powerful, corrosive acid. The strong acids are hydrochloric acid, nitric acid, sulfuric acid, hydrobromic acid, hydroiodic acid, perchloric acid, and chloric acid. This makes hf the only hydrohalic acid. It's corrosive to bones because of its fluoride. The only weak acid formed by the reaction. Hydrofluoric acid or hf is an extremely corrosive. Is Hf Strong Or Weak Acid.
From www.youtube.com
Why hydrogen fluoride is a weaker acid than hydrochloric acid Find Is Hf Strong Or Weak Acid The only weak acid formed by the reaction. Hf is corrosive because of its proton; Hc 2 h 3 o 2. It's corrosive to bones because of its fluoride. This makes hf the only hydrohalic acid. Hydrofluoric acid or hf is an extremely powerful, corrosive acid. However, it is a weak acid and not a strong acid because. Any acid. Is Hf Strong Or Weak Acid.
From www.slideserve.com
PPT Ionization Constants of Acids and Bases Strengths of Acids and Is Hf Strong Or Weak Acid Hc 2 h 3 o 2. Hf corrodes glass and hcl doesn't because silicon. Hydrofluoric acid or hf is an extremely powerful, corrosive acid. Hydrofluoric acid is a weak acid compared to many others, but its health risks are particularly severe due to its ability to penetrate tissue. Any acid that dissociates 100% into ions is called a strong acid.. Is Hf Strong Or Weak Acid.
From www.slideserve.com
PPT Common Strong acids PowerPoint Presentation, free download ID Is Hf Strong Or Weak Acid This makes hf the only hydrohalic acid. However, it is a weak acid and not a strong acid because. Hydrofluoric acid or hf is an extremely powerful, corrosive acid. The strong acids are hydrochloric acid, nitric acid, sulfuric acid, hydrobromic acid, hydroiodic acid, perchloric acid, and chloric acid. Hf is corrosive because of its proton; Hydrofluoric acid or hf is. Is Hf Strong Or Weak Acid.
From shiken.ai
Weak Acids and Bases Is Hf Strong Or Weak Acid Hydrofluoric acid is a weak acid compared to many others, but its health risks are particularly severe due to its ability to penetrate tissue. However, it’s classified as a weak acid rather than a strong acid. Hf corrodes glass and hcl doesn't because silicon. However, it is a weak acid and not a strong acid because. Hf is corrosive because. Is Hf Strong Or Weak Acid.
From www.numerade.com
SOLVED HCI (aq) is a strong acid, which means that HCl is a stronger Is Hf Strong Or Weak Acid If it does not dissociate 100%, it is a weak acid. Hc 2 h 3 o 2. The only weak acid formed by the reaction. However, it’s classified as a weak acid rather than a strong acid. Any acid that dissociates 100% into ions is called a strong acid. Hydrofluoric acid or hf is an extremely corrosive acid. Hf corrodes. Is Hf Strong Or Weak Acid.
From topblogtenz.com
Is HF an acid or base? Strong or Weak Hydrogen fluoride Is Hf Strong Or Weak Acid Hc 2 h 3 o 2. It's corrosive to bones because of its fluoride. Hydrofluoric acid or hf is an extremely powerful, corrosive acid. However, it is a weak acid and not a strong acid because. Hf is corrosive because of its proton; The strong acids are hydrochloric acid, nitric acid, sulfuric acid, hydrobromic acid, hydroiodic acid, perchloric acid, and. Is Hf Strong Or Weak Acid.
From www.pearson.com
HF is a weak electrolyte and HBr is a strong electrolyte. Which o Is Hf Strong Or Weak Acid It's corrosive to bones because of its fluoride. The only weak acid formed by the reaction. Any acid that dissociates 100% into ions is called a strong acid. However, it’s classified as a weak acid rather than a strong acid. Hydrofluoric acid or hf is an extremely corrosive acid. If it does not dissociate 100%, it is a weak acid.. Is Hf Strong Or Weak Acid.
From inspiritvr.com
Strong and Weak Acids and Acid Ionization Constant (Ka) Inspirit Is Hf Strong Or Weak Acid Hc 2 h 3 o 2. Hydrofluoric acid or hf is an extremely corrosive acid. However, it’s classified as a weak acid rather than a strong acid. Hf is corrosive because of its proton; The strong acids are hydrochloric acid, nitric acid, sulfuric acid, hydrobromic acid, hydroiodic acid, perchloric acid, and chloric acid. Hf corrodes glass and hcl doesn't because. Is Hf Strong Or Weak Acid.
From slideplayer.com
8.3 Strong vs. Weak Acid & Base Titrations ppt download Is Hf Strong Or Weak Acid Hf corrodes glass and hcl doesn't because silicon. Hydrofluoric acid is a weak acid compared to many others, but its health risks are particularly severe due to its ability to penetrate tissue. It's corrosive to bones because of its fluoride. However, it’s classified as a weak acid rather than a strong acid. Hydrofluoric acid or hf is an extremely powerful,. Is Hf Strong Or Weak Acid.
From www.ck12.org
Weak Acids and Bases Overview ( Video ) Chemistry CK12 Foundation Is Hf Strong Or Weak Acid Hydrofluoric acid or hf is an extremely powerful, corrosive acid. It's corrosive to bones because of its fluoride. Hf corrodes glass and hcl doesn't because silicon. Hydrofluoric acid or hf is an extremely corrosive acid. If it does not dissociate 100%, it is a weak acid. However, it’s classified as a weak acid rather than a strong acid. The only. Is Hf Strong Or Weak Acid.
From www.numerade.com
SOLVED acid strong or weak? species present at 106 mol/L or greater Is Hf Strong Or Weak Acid Hydrofluoric acid or hf is an extremely corrosive acid. However, it’s classified as a weak acid rather than a strong acid. If it does not dissociate 100%, it is a weak acid. Hf is corrosive because of its proton; Hf corrodes glass and hcl doesn't because silicon. Hydrofluoric acid is a weak acid compared to many others, but its health. Is Hf Strong Or Weak Acid.
From www.numerade.com
SOLVED The chemical formulae of some acids are listed in the first Is Hf Strong Or Weak Acid It's corrosive to bones because of its fluoride. This makes hf the only hydrohalic acid. Hf is corrosive because of its proton; The strong acids are hydrochloric acid, nitric acid, sulfuric acid, hydrobromic acid, hydroiodic acid, perchloric acid, and chloric acid. However, it is a weak acid and not a strong acid because. Any acid that dissociates 100% into ions. Is Hf Strong Or Weak Acid.
From www.slideshare.net
1 22 What Are Strong Acids And Bases Is Hf Strong Or Weak Acid Hf is corrosive because of its proton; If it does not dissociate 100%, it is a weak acid. Any acid that dissociates 100% into ions is called a strong acid. Hf corrodes glass and hcl doesn't because silicon. Hc 2 h 3 o 2. This makes hf the only hydrohalic acid. Hydrofluoric acid is a weak acid compared to many. Is Hf Strong Or Weak Acid.
From www.teachoo.com
List of Strong Acids (More than 8 examples, with images) Teachoo Is Hf Strong Or Weak Acid It's corrosive to bones because of its fluoride. Hf corrodes glass and hcl doesn't because silicon. If it does not dissociate 100%, it is a weak acid. However, it is a weak acid and not a strong acid because. The only weak acid formed by the reaction. Hydrofluoric acid or hf is an extremely corrosive acid. Hc 2 h 3. Is Hf Strong Or Weak Acid.
From www.youtube.com
Is HF (Hydrofluoric acid) an Electrolyte or NonElectrolyte? YouTube Is Hf Strong Or Weak Acid Hydrofluoric acid or hf is an extremely powerful, corrosive acid. However, it is a weak acid and not a strong acid because. The strong acids are hydrochloric acid, nitric acid, sulfuric acid, hydrobromic acid, hydroiodic acid, perchloric acid, and chloric acid. The only weak acid formed by the reaction. Hydrofluoric acid is a weak acid compared to many others, but. Is Hf Strong Or Weak Acid.
From www.chegg.com
Solved Which Diagram Represents An Aqueous Solution Of HF... Is Hf Strong Or Weak Acid However, it is a weak acid and not a strong acid because. If it does not dissociate 100%, it is a weak acid. Any acid that dissociates 100% into ions is called a strong acid. Hf is corrosive because of its proton; Hydrofluoric acid or hf is an extremely corrosive acid. Hc 2 h 3 o 2. The only weak. Is Hf Strong Or Weak Acid.
From www.slideserve.com
PPT Chapter 18 PowerPoint Presentation, free download ID1302194 Is Hf Strong Or Weak Acid Hc 2 h 3 o 2. However, it is a weak acid and not a strong acid because. If it does not dissociate 100%, it is a weak acid. Hf is corrosive because of its proton; Hydrofluoric acid or hf is an extremely powerful, corrosive acid. This makes hf the only hydrohalic acid. It's corrosive to bones because of its. Is Hf Strong Or Weak Acid.
From slideplayer.com
Introduction to Acids and Bases ppt download Is Hf Strong Or Weak Acid If it does not dissociate 100%, it is a weak acid. It's corrosive to bones because of its fluoride. Hf corrodes glass and hcl doesn't because silicon. The strong acids are hydrochloric acid, nitric acid, sulfuric acid, hydrobromic acid, hydroiodic acid, perchloric acid, and chloric acid. Hydrofluoric acid or hf is an extremely corrosive acid. Hydrofluoric acid or hf is. Is Hf Strong Or Weak Acid.
From www.numerade.com
SOLVED 4 of 40 Part A Classify the following compounds as weak acids Is Hf Strong Or Weak Acid If it does not dissociate 100%, it is a weak acid. Any acid that dissociates 100% into ions is called a strong acid. However, it’s classified as a weak acid rather than a strong acid. However, it is a weak acid and not a strong acid because. It's corrosive to bones because of its fluoride. Hc 2 h 3 o. Is Hf Strong Or Weak Acid.
From www.transtutors.com
(Solved) Strong Acid HF Weak Acid HI Strong Base NaClO4 Weak Base NaI Is Hf Strong Or Weak Acid However, it’s classified as a weak acid rather than a strong acid. Any acid that dissociates 100% into ions is called a strong acid. Hc 2 h 3 o 2. If it does not dissociate 100%, it is a weak acid. Hf corrodes glass and hcl doesn't because silicon. Hydrofluoric acid or hf is an extremely powerful, corrosive acid. It's. Is Hf Strong Or Weak Acid.
From blog.praxilabs.com
Learn All About The Strong Acids and Bases PraxiLabs Is Hf Strong Or Weak Acid Hydrofluoric acid or hf is an extremely powerful, corrosive acid. Hydrofluoric acid or hf is an extremely corrosive acid. The only weak acid formed by the reaction. Hc 2 h 3 o 2. Hydrofluoric acid is a weak acid compared to many others, but its health risks are particularly severe due to its ability to penetrate tissue. However, it’s classified. Is Hf Strong Or Weak Acid.
From danielafersmedina.blogspot.com
Hbro3 Strong or Weak Acid or Base Is Hf Strong Or Weak Acid If it does not dissociate 100%, it is a weak acid. Any acid that dissociates 100% into ions is called a strong acid. However, it’s classified as a weak acid rather than a strong acid. Hydrofluoric acid or hf is an extremely powerful, corrosive acid. Hf corrodes glass and hcl doesn't because silicon. Hf is corrosive because of its proton;. Is Hf Strong Or Weak Acid.
From www.numerade.com
SOLVED Classify each of the following as a strong acid or a weak acid Is Hf Strong Or Weak Acid Any acid that dissociates 100% into ions is called a strong acid. Hydrofluoric acid is a weak acid compared to many others, but its health risks are particularly severe due to its ability to penetrate tissue. This makes hf the only hydrohalic acid. Hc 2 h 3 o 2. It's corrosive to bones because of its fluoride. The strong acids. Is Hf Strong Or Weak Acid.
From www.numerade.com
SOLVED Texts Module 9 Homework Identify whether the following are Is Hf Strong Or Weak Acid Hc 2 h 3 o 2. Hydrofluoric acid or hf is an extremely corrosive acid. The only weak acid formed by the reaction. This makes hf the only hydrohalic acid. It's corrosive to bones because of its fluoride. Hydrofluoric acid or hf is an extremely powerful, corrosive acid. The strong acids are hydrochloric acid, nitric acid, sulfuric acid, hydrobromic acid,. Is Hf Strong Or Weak Acid.
From www.numerade.com
SOLVED Identify hydrofluoric acid strong electrolyte, strong acid weak Is Hf Strong Or Weak Acid However, it’s classified as a weak acid rather than a strong acid. This makes hf the only hydrohalic acid. The only weak acid formed by the reaction. It's corrosive to bones because of its fluoride. If it does not dissociate 100%, it is a weak acid. Any acid that dissociates 100% into ions is called a strong acid. Hydrofluoric acid. Is Hf Strong Or Weak Acid.
From www.thoughtco.com
List of Common Strong and Weak Acids Is Hf Strong Or Weak Acid This makes hf the only hydrohalic acid. However, it is a weak acid and not a strong acid because. Any acid that dissociates 100% into ions is called a strong acid. Hc 2 h 3 o 2. It's corrosive to bones because of its fluoride. The only weak acid formed by the reaction. Hydrofluoric acid or hf is an extremely. Is Hf Strong Or Weak Acid.
From www.thoughtco.com
Is Hydrofluoric Acid (HF) a Strong or Weak Acid? Is Hf Strong Or Weak Acid However, it is a weak acid and not a strong acid because. It's corrosive to bones because of its fluoride. Hf is corrosive because of its proton; However, it’s classified as a weak acid rather than a strong acid. The only weak acid formed by the reaction. Hf corrodes glass and hcl doesn't because silicon. If it does not dissociate. Is Hf Strong Or Weak Acid.
From www.numerade.com
SOLVEDExplain why HF is a weak acid, whereas HCl, HBr, and HI are all Is Hf Strong Or Weak Acid However, it’s classified as a weak acid rather than a strong acid. Hydrofluoric acid is a weak acid compared to many others, but its health risks are particularly severe due to its ability to penetrate tissue. Hydrofluoric acid or hf is an extremely corrosive acid. However, it is a weak acid and not a strong acid because. The only weak. Is Hf Strong Or Weak Acid.
From www.teachoo.com
Examples of Weak Acids 5+ Examples Teachoo Teachoo Questions Is Hf Strong Or Weak Acid It's corrosive to bones because of its fluoride. Hydrofluoric acid or hf is an extremely corrosive acid. Any acid that dissociates 100% into ions is called a strong acid. However, it is a weak acid and not a strong acid because. Hydrofluoric acid is a weak acid compared to many others, but its health risks are particularly severe due to. Is Hf Strong Or Weak Acid.
From www.slideserve.com
PPT AcidBase Reactions PowerPoint Presentation, free download ID Is Hf Strong Or Weak Acid Hc 2 h 3 o 2. If it does not dissociate 100%, it is a weak acid. However, it’s classified as a weak acid rather than a strong acid. It's corrosive to bones because of its fluoride. Hf is corrosive because of its proton; However, it is a weak acid and not a strong acid because. Hydrofluoric acid or hf. Is Hf Strong Or Weak Acid.
From www.youtube.com
Why is hydrofluoric acid, HF, a weak acid compared to other hydrogen Is Hf Strong Or Weak Acid The strong acids are hydrochloric acid, nitric acid, sulfuric acid, hydrobromic acid, hydroiodic acid, perchloric acid, and chloric acid. If it does not dissociate 100%, it is a weak acid. Hydrofluoric acid or hf is an extremely powerful, corrosive acid. Hydrofluoric acid is a weak acid compared to many others, but its health risks are particularly severe due to its. Is Hf Strong Or Weak Acid.